Abstract
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that affects memory, thinking, attention, and emotion or AD. Smelling problems are frequent symptoms of dementia. The aim of this study was to evaluate whether it is possible to predict if someone with anosmia or hyposmia has a higher risk of getting dementia or even AD.
Methods
This study was a retrospective longitudinal study, and the data used were part of a larger research project, the Vienna Conversion to Dementia Study. The 173 participants were divided into four groups based on cognitive features such as healthy control (HC), subjective cognitive decline (SCD), non-amnestic mild cognitive impairment (naMCI), and amnestic mild cognitive impairment (aMCI). Olfactory assessment and neurocognitive assessment were administered.
Results
We found that 30.5% of aMCI patients converted into AD after an average of about two years. The corresponding ROC analyses for olfactory testing showed that Sniffin’ Sticks revealed significant results regarding the conversion to AD, whereas the Assessment of Self-Reported Olfactory Functioning and olfaction-related quality of life (ASOF) inventory using the Subjective Olfactory Capability (SOC) subscale, the Smell-Related Problems (SRP) subscale, and the Olfaction-Related Quality of life (ORQ) did not. A logistic regression showed that among the olfactory test procedures, only the Sniffin’ Sticks enabled a relevant prognosis. Including neurocognitive measures in the model, only VSRT and the Trail Making Test-B. The other predictors did not contribute to the prediction of conversion to AD.
Conclusion
Unlike self-reporting of olfactory functioning, olfactory testing using standardized tests may have potential for predicting dementia, especially AD. However, olfactory tests have lower predictive power than neurocognitive tests such as verbal memory and divided attention tests.
Implications
Diagnostic tools for predicting dementia as accurately and early as possible are important. Olfactory assessment, compared to neurocognitive tests for verbal memory and divided attention, is inferior in predicting the prognosis of AD.
Similar content being viewed by others
Introduction
Olfactory deficits have been found to be relatively prevalent in large population-based studies (Yang and Pinto 2016). They occur as either qualitative or quantitative impairments occur due to various etiologies, including trauma, viral upper respiratory tract infections, nasal or sinus disease, and neurodegenerative diseases. Among the neurodegenerative diseases, it is not only Alzheimer’s disease (AD) that displays olfactory dysfunction but also other forms of dementia like vascular dementia, Parkinson’s disease, and frontotemporal dementia (Alves et al. 2014). The prevalence of olfactory dysfunction is estimated to be 3.8% in adults between the ages of 21 and 84 and to increase with age, from 0.6% in those < 35 years to 13.9% among those ≥ 65 years. There is a higher prevalence in men (Schubert et al. 2012).
There are differences between AD and other dementias when it comes to a decrease in olfactory function. For one Olfactory function is highly impaired in AD. More precisely, it occurs in 100% of these patients, compared to 96% of patients with frontotemporal dementia (Pardini et al. 2009) and about 15% of patients with vascular dementia (Duff et al. 2002). Patients with Parkinson’s disease show similar impairment as AD patients (Chou and Bohnen 2009).
Years before AD is diagnosed, patients can experience several preclinical stages, which can start with subjective cognitive decline (SCD) not yet severe enough to be measured in objective memory tests. The next stage begins when patients suffer mild cognitive impairment (MCI). Unlike patients with impairments in cognitive function, participants with normal cognitive function are aware of subtle memory deficits. These are just not yet severe enough to be measured in objective memory tests. Therefore, subjective memory complaints can predict cognitive decline only in individuals with normal baseline cognition (Geerlings et al. 1999; Murphy and Levine 2010; Wehling et al. 2011). There are theories that the frontal lobe can compensate and help retain objective memory performance (Erk et al. 2011). Thus, these findings demonstrate that subjective memory complaints are early markers of dementia in the elderly population, despite the apparently low reliability of memory self-assessments, and this might also be true when it comes to complaints about olfactory function (Stanciu et al. 2014).
The question of interest is whether participants within the normal rage of cognitive function, but in a preclinical stage of a dementia form, are able to notice olfactory deficits by themselves. The awareness of a dysfunction and the knowledge of its importance could influence an individual to see a doctor and possibly take precautions. There are few studies that have investigated self-assessed olfactory impairments among elderly participants, and these have found a low correlation between self-assessment of olfactory ability and real performance on standardized tests of olfactory functions (Stanciu et al. 2014).
When participants without generalized cognitive impairment rated their sense of smell as “worse than normal,” they had 2.17 times the odds of dementia conversion within a 10-year timespan. The effect present not only in AD but also in other dementia forms like vascular dementia and remained stable after controlling for demographic variables, cognitive ability, odor identification ability, and symptoms of depression. These results showed that subjective olfactory complaints may play a role in the early detection of dementia and that a multi-factorial approach to predicting and evaluating dementia would have significant impact (Schmand et al. 1996; Stanciu et al. 2014; Alveset al. 2014).
A study from Tahmasebi et al. (2019) analyzed the relationship between olfactory and semantic memory impairment among participants with SCD or (MCI) at baseline. The results showed that for olfactory impairment, only the Sniffin’ Sticks tests were significantly different between converted and non-converted participants, indicating that converted participants had a lower odor-identification ability than the other group (Tahmasebi et al. 2019). Olfactory function is often impaired in participants with AD, particularly in patients with mild cognitive impairment amnestic type (aMCI), which could be used as a predictive marker for AD (Conti et al. 2013; Djordjevic et al. 2008; Fusetti et al. 2010).
Tahmasebi et al. (2019) also showed that objective olfactory assessments were highly useful for prediction of conversion to AD among MCI participants, but their low sensitivity appeared to be a problem. Thus, combining them with a neuropsychological test would be far more useful for the estimation of the risk of AD development (Tahmasebi et al. 2019).
The aim of this study was to evaluate whether patients with anosmia or hyposmia have a higher risk of converting to AD. Furthermore, we were interested in determining if methods for evaluating olfactory ability and the self-assessment of smelling capability are useful in making predictions about AD risks.
Material and Methods
Patients
This study was a retrospective analysis of data collected by the Vienna Conversion to Dementia Study at the Department of Neurology of the Medical University of Vienna between 2008 and 2017. Data collection was performed in accordance with the Helsinki Declaration and was approved by the Ethical Committee of the Medical University of Vienna. Patients either were referred to the study site by physicians or were self-referrals. Patients underwent underwent a neurological examination and extensive neuropsychological testing. Patients were excluded from the study if any of the following conditions applied: age younger than 50 years; evidence of stroke as defined by neuroradiological and clinical examination; a history of severe head injury or current psychiatric diagnoses, excluding patients with sub-depressive symptoms that often occur in elderly patients according to the International Classification of Diseases (ICD)-10 (Dilling et al. 2015); or any medical condition associated with severe cognitive deterioration, including renal, respiratory, cardiac, and hepatic disease.
One hundred seventy-four participants were divided into four groups based on cognitive features, as follows: SCD, aMCI, on-amnestic mild cognitive impairment (naMCI), and AD. An SCD classification required the presence of subjective memory deterioration, manifested by the seeking of medical help for memory problems, and the concurrent absence of any objectively measurable cognitive deficits (Jessen et al. 2014). aMCI was determined by a mean z-score for the memory domain below1.5 SD, and naMCI was determined by a mean z-score in at least one cognitive domain other than the memory domain below SD (Pusswald et al. 2013). The diagnosis of AD was determined by a consensus committee that included a neuropsychologist, a neurologist, and other study personnel who were involved in the evaluation of the patients’ cognitive status. AD was diagnosed according to criteria established by the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (Mckhann et al. 1984) and the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (Sass 2003). Cognitively healthy control participants were recruited by means of advertisements. Control participants underwent a rigorous evaluation involving a standardized clinical interview and cognitive screening.
Demographic and clinical characteristics are shown in Table 1.
Psychometric Testing
Neuropsychological Assessment
The Wortschatztest (WST) (Schmidt 1992) was used to estimate premorbid verbal intelligence, the Beck Depression Inventory (BDI-II)(Hautzinger et al. 2006) was conducted for assessment of depression and the Mini-Mental State Examination (MMSE) (Folstein et al. 1975) was used as a screening tool. The Neuropsychological Test Battery Vienna (NTBV) (Sass 2003) was administered to assess cognitive function. This battery includes several tests that examine domains of attention, executive functioning, language, and memory (Lehrner 2007). Attention was assessed using the Alters-Konzentrationstest (AKT) (Gatterer 1990), a geriatric cancelation test; the digit symbol subtest of German Wechsler Adult Intelligence Scale (WAIS-R); the symbol counting subtest of the cerebral insufficiency test (C.I.) (Lehrl 1999); and the Stroop Test from the Nürnberger Alters-Inventar (NAI) (Oswald and Fleischmann 1993). Psychomotor speed was examined using the symbol counting subtest of the C.I. Semantic verbal fluency was assessed by having patients name as many animals, supermarket items, and tools as possible within one minute each. Naming as many words as possible beginning with the letters b, f, and I each within one minute, was used as a measure of lexical verbal fluency. The Boston Naming Test (mBNT) assessed naming performance in patients (Goodglass et al. 2001). Episodic memory was tested using the Verbal Selective Reminding Test (VSRT) (Lehrner et al. 2006). Trail Making Test B (TMT) was administered to assess divided attention. The score difference between Parts A and B of the TMT (Reitan 1958), the Five-Point Test (Regard et al. 1982), and the Maze Test from the NAI Test Battery were used to examine executive functions.
Odor Assessment
All participants filled in a 12-item questionnaire for the assessment of self-reported olfactory functioning and olfaction-related quality of life (ASOF) (Pusswald et al. 2012; Psimistri, 2022). This tool can be subdivided into three domains: the one-item, subjective olfactory capability (SOC) scale, with a cut-off score of > 3; the five-item self-reported capability of perceiving specific odors (SRP) scale, with a cut-off score of < 2.9, and the six-item olfactory-related quality of life (ORQ) scale, with a cut-off score of > 3.7. Each subject’s olfactory function was assessed using the Sniffin’ Sticks odor identification test (OIT), with a cut-off score of 10 (Hummel et al. 1997).
Procedure
The participants underwent these assessments twice (at baseline T1 and at time point T2). The interval between examinations at T1 and T2 was between 12 and 48 months.
Statistical Analysis
The statistical calculations were performed with the IBM SPPS® Version 25 analysis software. The significance level within the context of inferential statistics was set at α = 5%. The standardized effect size \(r\) according to Cohen’s classification was used to assess the relevance of results in terms of content. The odds ratio (OR) was calculated as an approximation of relative risk.
The distribution of parametric variable mean (M) and standard deviation for skewed distributions median (Md) and interquartile range (IQR) were calculated. For proportional values of nominally scaled variables, the corresponding confidence interval [95%-CI: lower level, LL%; upper level, UL%] was also calculated using the expression. For the 5% error probability of the CI, the corresponding bilateral z value of 1.96 was used.
We calculated the Kruskal–Wallis (KW) test and the Mann–Whitney \(U\)-test for comparison and the one-way analysis of variance (ANOVA) for normal distribution data. The χ2 test was applied for distribution analysis. Furthermore, a binary logistic regression was used as a multiple method in the context of model testing as prognosis to differentiate between AD and non-AD patients. We used the significant parameters among those used for logistic regressions for receiver-operating characteristic (ROC) analyses.
Results
Table 1 shows sociodemographic and neurocognitive variables, NTBV subtests, and olfactory performance depending on the participants' (patients') categories at baseline (time point T1). Analyses revealed significant differences in age and years of education. Post hoc pairwise procedures according to Bonferroni revealed that the participants in the healthy control group (HC) were younger than in the other groups. On average, participants in the HC also had more years of education than the other groups (p’s ≤ 0.001). The analyses also showed significant differences in MMSE, BDI-II, and WST among the participant groups at T1. Test results revealed significant differences in all NTBV subtests among the participant groups at T1 except on the Five Point Test preserve. Furthermore, significant differences in Sniffin’ Sticks performance and ASOF ORQ scores among the participant groups at T1 were found.
Of the 173 participants, a total of 95 (54.9%) had the same diagnosis at both times, whereas 44 (25.4%) were shown to be worsening, and 34 (19.7%) showed an improvement. The results of χ2 = 1.282, p = 0.308 demonstrated no significance. Specifically, 18 of the 59 aMCI (30.5%; 95% CI [18.8%; 42.3%]) patients showed conversion to AD after an average of two years, whereas the other 18 of the 59 aMCI showed an improvement, 13 (7.5%) toward naMCI, and 5 (2.9%) toward SCD. Additionally, there was no conversion of the SCD and naMCI patients to AD. As a result, we were able to categorize 41 patients as non-AD and the remaining 18 as AD.
Table 2 shows the results of relevant olfactory performance and neuropsychological subtests at time point two (T2) depending on the different diagnostic groups within the sample. The examination of group differences in olfactory function at T2 was carried out by KW testing. Test results revealed significant differences in all subtests of the neuropsychological test-battery among the participant groups at T2 except the Five Point Test preserve as well as Sniffin’ Sticks and ASOF SOC scores.
Model testing using binary and logistic regression was performed to predict the AD criterion. Initially, the explanatory values of the Sniffin’ Sticks and ASOF subtests were examined using the stepwise backward method in order to identify those tests that made a meaningful contribution. The Hosmer–Lemeshow test indicated model fit, p = 0.71. Table 3 shows the results for the prediction of the AD criterion. It should be noted that 14 participants were not included in this analysis because they did not have complete records of olfactory performance at T1. Therefore, a sample size of n = 159 was used for this binary logistic regression.
The result showed that only the Sniffin’ Sticks (p = 0.01, OR = 0.73; 95% CI [0.61; 0.88], R2 = 17.7%) enabled a relevant prognosis.
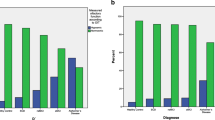
The corresponding ROC analyses for olfactory testing at T1 revealed that Sniffin’ Sticks outperformed the ASOF regarding the prediction of conversion to AD (see Fig. 1 for details).
The corresponding ROC analyses for Sniffin’ sticks and ASOF subtests at baseline (T1). Subjective Olfactory Capability scale (SOC)-Subjektives Riechvermögen; SOC (self-reported capability of perceiving specific odors scale (SRP)-Beeinträchtigung der Wahrnehmung von Alltagsdüften; (olfactory-related quality of life (ORQ)-Riechbezogene Lebensqualität
In the next step, the explanatory value of the NTBV subtests was examined using the stepwise backward method in order to identify those tests that made a meaningful contribution to the prediction of conversion to AD.
A total of six subtests (AKT TT, C.I. Symbols, TMT B, Phonematische Wortflussigkeit (PWT), VSRT total, and VSRT recognition) showed a significant effect on the prediction of the conversion to AD. See Table 4 for details.
A logistic regression showed that among the olfactory test procedures, only the Sniffin’ Sticks enabled a relevant prognosis. Therefore, we did not include the ASOF subtests in the following analysis. The significant NTBV subtests were then subjected to this further model, together with olfactory performance (Sniffin’ Sticks) and sociodemographic characteristics (sex, age, and education years).
The weight of the first block was 15.0%, including only Sniffin’ Sticks. The first and second block had a combined weight of 19.6%, with the sociodemographic variables included. The additional inclusion of the NTBV subtest services increased the prognosis resulting in a declared variance level of 72.4%. "Risk for AD was indicated by low scoring on the learning subtest of the VSRT (p = 0.01, OR = 0.83; 95% CI [0.73; 0.94]), recognition (p = 0.01, OR = 0.67; 95% CI [0.45; 0.996]), and higher values for divided attention (TMT B) (p = 0.01, OR = 1.03; 95% CI [1.01; 1.05]) indicated a risk for AD. The other predictors did not contribute to a correct prognosis for AD.
The corresponding ROC analyses for the relevant neuropsychological subtests at T1 showed that the subtests of the VSRT, Learning and Recognition, and the TMT B for testing divided attention showed satisfactory validity regarding the prognosis of AD (see Fig. 2 for details).
Visual inspection shows that the neurocognitive measures are outperforming the olfactory measures in terms of predicting AD.
Discussion
In the present study, the data protocols of 173 participants, aged ≥ 50 years, were analyzed. The main objectives were to examine the prognostic validity of neuropsychological and odor assessment tools with regard to conversion into AD. We wanted to answer the question of whether someone with anosmia has a higher risk of dementia and whether Sniffin’ Sticks and ASOF can predict the risk. We examined whether age, sex, and years of education as covariates, together with the applied neurocognitive tests, could provide a prognosis of the probability of the conversion to AD.
Participant groups showed differences in age and years of education; HC were younger than in the other groups and had more education. The analysis also showed differences in the MMSE, BDI II, and WST among the participant groups at T1. Results of olfactory function procedures at T1 depending on the different diagnostic groups within the sample indicated differences in Sniffin’ Sticks performance and ORQ, part of the ASOF questionnaire.
With regard to olfactory performance, the patient groups at T2 differed only on the subtests of the ASOF questionnaire in SRP and ORQ, whereas participant groups did not differ on Sniffin’ Sticks and SOC results. Again, test results revealed significant differences in all NTBV subtests among the participant groups at T2, except perseveration errors in the Five Point Test. The remaining subtests of the NTBV were then subjected to a further model analysis, together with the olfactory performance (Sniffin’ Sticks) and the sociodemographic characteristics (sex, age, and education years). The additional inclusion of the NTBV subtests with the Sniffin’ Sticks and sociodemographic variables increased the predictive validity. Risk for AD was predicted by low scoring in verbal memory, and low attention. The other predictors did not contribute to the prognosis for AD.
The corresponding ROC analyses for olfactory testing showed that Sniffin’ Sticks had a satisfactory validity regarding the prognosis of AD, whereas the ASOF measures showed low validity. Compared to the Sniffin' Sticks, the NTBV subtests for memory and attention showed superior predictive validity in the ROC analysis.
Jessen et al. (2014) already focused on predictors of AD and found that several studies used gender, age, the mini-mental status test (MMSE), individual CSF biomarker data, MRI-based hippocampal volumes, model to make predictions. The studies showed that gender and age did not provide an accurate prediction of dementia risk (Rostamzadeh and Jessen 2020). This is consistent with our results, which showed that gender and age do not make an impact.
Our results supported the results of the study by Tahmasebi et al. (2019). That study showed that the Sniffin’ Sticks performance was significantly lower in AD patients compared to healthy controls, SCD, naMCI, and aMCI patients; significantly lower in aMCI patients compared to healthy controls, SCD, and naMCI patients; and significantly lower in naMCI patients compared to healthy controls. The Sniffin’ Sticks also produced significant results in the ROC analyses (Tahmasebi et al. 2019).
Stanciu et al. (2014) also conducted a logistic regression as the main analysis, in which age, gender, years of education, MMSE score, olfactory identification performance, and reporting olfactory impairment were used as predictor variables and conversion to dementia, with a period of 10 years, as the criterion variable. They showed that after adjustment for demographic variables and MMSE scores, olfactory identification and subjective olfactory impairment significantly and independently predicted conversion to dementia. Patients who rated their sense of smell as “worse than normal” were more likely to be diagnosed with dementia within 10 years than patients who did not report olfactory impairment. Adding reports of olfactory complaints in the last step of the regression significantly improved the variance explained by the logistic model and increased the the accuracy of dementia predictions.
This improvement of explained variance through reporting olfactory complaints could not be shown in our results. However, gender and years of education had no independent influence on the probability of conversion to dementia, just as our results showed. It is worth mentioning that Stanciu et al.'s (2014) study examined participants over 10 years. In our study, the time interval of examination was on average 24 months.
Limitations and Preview
One limitation that should be mentioned is that participant groups showed differences in age, education years, and some basic clinical data. This fact should not be ignored when discussing the results. The study was also limited due to the circumstance that only those test protocols that were completely available on the two examination dates could be considered. Furthermore, it should be noted that the patient sample at T2 had a comparatively small number of AD patients.
Conclusion
Using a retrospective longitudinal study design, we examined the extent to which standard neurocognitive diagnostic procedures provide prognostic value in the correct identification of AD patients when combined with olfactory measures. Sniffin’ Sticks were identified as a measure with satisfactory predictive validity for conversion to AD. However, predictive validity disappeared when used together with standard neurocognitive measures of memory and attention. In conclusion, olfactory assessment might be a useful tool in assessing conversion to dementia but might be inferior predicting to conversion to dementia compared to standard neurocognitive measures of memory and attention.
Change history
24 April 2023
Change of article title.
References
Alves J, Petrosyan A, Magalhaes R (2014) Olfactory dysfunction in dementia. World J Clin Cases 11:661–667. https://doi.org/10.12998/wjcc.v2.i11.661
Chou KL, Bohnen NI (2009) Performance on an Alzheimer-selective odor identification test in patients with Parkinson’s disease and its relationship with cerebral dopamine transporter activity. Parkinsonism Relat Disord 9:640–643. https://doi.org/10.1016/j.parkreldis.2009.03.004
Conti MZ, Vicini-Chilovi B, Riva M, Zanetti M, Liberini P, Padovani A, Rozzini L (2013) Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer’s disease. Arch Clin Neuropsychol 5:391–399. https://doi.org/10.1093/arclin/act032
Dilling H, Mombour W,Schmidt M. (2015) Internationale Klassifikation psychischer Störungen: ICD–10 Kapitel V (F) – Klinisch–diagnostische Leitlinien Hogrefe
Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H (2008) Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 5:693–706. https://doi.org/10.1016/j.neurobiolaging.2006.11.014
Duff K, McCaffrey RJ, Solomon GS (2002) The Pocket Smell Test: successfully discriminating probable Alzheimer’s dementia from vascular dementia and major depression. J Neuropsychiatry Clin Neurosci 2:197–201. https://doi.org/10.1176/jnp.14.2.197
Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F (2011) Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry 8:845–852. https://doi.org/10.1001/archgenpsychiatry.2011.80
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res 3:189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Fusetti M, Fioretti AB, Silvagni F, Simaskou M, Sucapane P, Necozione S,Eibenstein A (2010) Smell and preclinical Alzheimer disease: study of 29 patients with amnesic mild cognitive impairment. Journal of otolaryngology - head & neck surgery = Le Journal d'oto-rhino-laryngologie et de chirurgie cervico-faciale. 2: 175–181
Gatterer G (1990) Alters-Konzentrations-Test (AKT). Verlag für Psychologie Hogrefe
Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B (1999) Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry 4:531–537. https://doi.org/10.1176/ajp.156.4.531
Goodglass H, Kaplan E,Baressi B. (2001) Boston Diagnostic Aphasia Examination. Psychological Corporation
Hautzinger M, Keller F,Kühner C. (2006) BDI-II Beck-Depressions-Inventar. Harcourt Test Services
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1:39–52. https://doi.org/10.1093/chemse/22.1.39
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M, Grp SIW (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers & Dementia 6:844–852. https://doi.org/10.1016/j.jalz.2014.01.001
Schmidt K-H (1992) Wortschatztest. Beltz Test
Lehrl S. (1999) Mehrfachwahl-Wortschatz-Intelligenztest: MWT-B. Spitta
Lehrner J, Gleiß A, Maly J, Auff E,Dal-Bianco P (2006) Der Verbale Selektive Reminding Test (VSRT). Neuropsychiatrie,. 204–214
Lehrner J (2007) The Neuropsychological Test Battery Vienna (NTBV). Psychologie in Österreich. 358–365
Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical-diagnosis of Alzheimers-disease - report of the Nincds-Adrda Work Group under the Auspices of Department-of-Health-and-Human-Services Task-Force on Alzheimers-Disease. Neurology 7:939–944. https://doi.org/10.1212/Wnl.34.7.939
Murphy MP, LeVine H 3rd (2010) Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis 1:311–323. https://doi.org/10.3233/JAD-2010-1221
Oswald W,Fleischmann U. (1993) Das Nürnberger Alters-Inventar NAI. Hogrefe Verlag
Pardini M, Huey ED, Cavanagh AL, Grafman J (2009) Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol 1:92–96. https://doi.org/10.1001/archneurol.2008.521
Psimistri (2022) Assessment of self-reported olfactory functioning and olfaction-related quality of life (ASOF), Manual, www.psimistri.com
Pusswald G, Auff E, Lehrner J (2012) Development of a brief self-report inventory to measure olfactory dysfunction and quality of life in patients with problems with the sense of smell. Chemosens Percept 3–4:292–299. https://doi.org/10.1007/s12078-012-9127-7
Pusswald G, Moser D, Gleiss A, Janzek-Hawlat S, Auff E, Dal-Bianco P, Lehrner J (2013) Prevalence of mild cognitive impairment subtypes in patients attending a memory outpatient clinic-comparison of two modes of mild cognitive impairment classification. Results of the Vienna Conversion to Dementia Study. Alzheimers & Dementia 4:366–376. https://doi.org/10.1016/j.jalz.2011.12.009
Regard M, Strauss E,Knapp P (1982) Children’s production on verbal and non-verbal fluency tasks. Percept Mot Skills. 3: 839–844
Reitan R (1958) The validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 271–276
Rostamzadeh A, Jessen F (2020) Early detection of Alzheimer’s disease and dementia prediction in patients with mild cognitive impairment: summary of current recommendations. Nervenarzt. https://doi.org/10.1007/s00115-020-00907-y
Sass H (2003) Diagnostisches und statistisches Manual psychischer Störungen. Hogrefe
Schmand B, Jonker C, Hooijer C, Lindeboom J (1996) Subjective memory complaints may announce dementia. Neurology 1:121–125. https://doi.org/10.1212/wnl.46.1.121
Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, Pankow JS, Nondahl DM (2012) Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chem Senses 4:325–334. https://doi.org/10.1093/chemse/bjr102
Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson LG, Olofsson JK (2014) Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc 2:209–217. https://doi.org/10.1017/s1355617713001409
Tahmasebi R, Zehetmayer S, Pusswald G, Kovacs G, Stogmann E, Lehrner J (2019) Identification of odors, faces, cities and naming of objects in patients with subjective cognitive decline, mild cognitive impairment and Alzheimer s disease: a longitudinal study. Int Psychogeriatr 4:537–549. https://doi.org/10.1017/s1041610218001114
Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ (2011) Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol 3:260–269. https://doi.org/10.1093/arclin/acr019
Yang J, Pinto JM (2016) The epidemiology of olfactory disorders. Curr Otorhinolaryngol Rep 2:130–141. https://doi.org/10.1007/s40136-016-0120-6
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interest, except for JL who is the CEO of psimistri GmbH which owns the website www.psimistri.com.
Ethics Approval and Consent
Participate All participants gave written informed consent.
The study was approved by the local Institutional Review Board (Medical University of Vienna) and was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pusswald, G., Ocak, S., Stögmann, E. et al. Odor identification testing is inferior compared to neurocognitive testing in predicting conversion to Alzheimer's Disease. Chem. Percept. 15, 185–193 (2022). https://doi.org/10.1007/s12078-022-09306-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-022-09306-w