Abstract
Introduction
Obesity is associated with increased breast cancer incidence, recurrence, and mortality. Adipocytes and adipose-derived stem cells (ASCs), two resident cell types in adipose tissue, accelerate the early stages of breast cancer progression. It remains unclear whether obesity plays a role in the subsequent escape of malignant breast cancer cells into the local circulation.
Methods
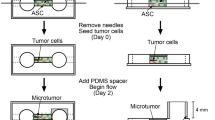
We engineered models of human breast tumors with adipose stroma that exhibited different obesity-specific alterations. We used these models to assess the invasion and escape of breast cancer cells into an empty, blind-ended cavity (as a mimic of a lymphatic vessel) for up to sixteen days.
Results
Lean and obese donor-derived adipose stroma hastened escape to similar extents. Moreover, a hypertrophic adipose stroma did not affect the rate of adipose-induced escape. When admixed directly into the model tumors, lean and obese donor-derived ASCs hastened escape similarly.
Conclusions
This study demonstrates that the presence of adipose cells, independently of the obesity status of the adipose tissue donor, hastens the escape of human breast cancer cells in multiple models of obesity-associated breast cancer.






Similar content being viewed by others
Abbreviations
- ASC:
-
Adipose-derived stem cell
- BMI:
-
Body mass index
- CM:
-
Conditioned medium
- ECM:
-
Extracellular matrix
- FFA:
-
Free fatty acid
- IFP:
-
Interstitial fluid pressure
- SMA:
-
Smooth muscle actin
- SVF:
-
Stromal-vascular fraction
References
Aherne, W. A., and M. S. Dunnill. Morphometry. London: Arnold Publishers, pp. 1–205, 1982.
Ahima, R. S., and J. S. Flier. Leptin. Annu. Rev. Physiol. 62:413–437, 2000.
Balaban, S., R. F. Shearer, L. S. Lee, M. van Geldermalsen, M. Schreuder, H. C. Shtein, R. Cairns, K. C. Thomas, D. J. Fazakerley, T. Grewal, J. Holst, D. N. Saunders, and A. J. Hoy. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 5:1, 2017.
Barone, I., C. Giordano, D. Bonofiglio, S. Andò, and S. Catalano. The weight of obesity in breast cancer progression and metastasis: clinical and molecular perspectives. Semin. Cancer Biol. 60:274–284, 2020.
Bousquenaud, M., F. Fico, G. Solinas, C. Rüegg, and A. Santamaria-Martínez. Obesity promotes the expansion of metastasis-initiating cells in breast cancer. Breast Cancer Res. 20:104, 2018.
Calle, E. E., and R. Kaaks. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4:579–591, 2004.
Chakarov, S., C. Blériot, and F. Ginhoux. Role of adipose tissue macrophages in obesity-related disorders. J. Exp. Med. 219:e20211948, 2022.
Cinti, S., G. Mitchell, G. Barbatelli, I. Murano, E. Ceresi, E. Faloia, S. Wang, M. Fortier, A. S. Greenberg, and M. S. Obin. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid. Res. 46:2347–2355, 2005.
Collins, J. M., M. J. Neville, K. E. Pinnick, L. Hodson, B. Ruyter, T. H. van Dijk, D. J. Reijngoud, M. D. Fielding, and K. N. Frayn. De novo lipogenesis in the differentiating human adipocyte can provide all fatty acids necessary for maturation. J. Lipid Res. 52:1683–1692, 2011.
Colpaert, C., P. Vermeulen, E. Van Marck, and L. Dirix. The presence of a fibrotic focus is an independent predictor of early metastasis in lymph node-negative breast cancer patients. Am. J. Surg. Pathol. 25:1557–1558, 2001.
Considine, R. V., M. K. Sinha, M. L. Heiman, A. Kriauciunas, T. W. Stephens, M. R. Nyce, J. P. Ohannesian, C. C. Marco, L. J. McKee, and T. L. Bauer. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334:292–295, 1996.
Cozzo, A. J., A. M. Fuller, and L. Makowski. Contribution of adipose tissue to development of cancer. Compr. Physiol. 8:237–282, 2017.
D’Esposito, V., F. Passaretti, A. Hammarstedt, D. Liguoro, D. Terracciano, G. Molea, L. Canta, C. Miele, U. Smith, F. Beguinot, and P. Formisano. Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia 55:2811–2822, 2012.
Dance, Y. W., T. Meshulam, A. J. Seibel, M. C. Obenreder, M. D. Layne, C. M. Nelson, and J. Tien. Adipose stroma accelerates the invasion and escape of human breast cancer cells from an engineered microtumor. Cell. Mol. Bioeng. 15:15–29, 2022.
Ewertz, M., M. B. Jensen, K. Gunnarsdóttir, I. Højris, E. H. Jakobsen, D. Nielsen, L. E. Stenbygaard, U. B. Tange, and S. Cold. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 29:25–31, 2011.
Fayanju, O. M., C. S. Hall, J. B. Bauldry, M. Karhade, L. M. Valad, H. M. Kuerer, S. M. DeSnyder, C. H. Barcenas, and A. Lucci. Body mass index mediates the prognostic significance of circulating tumor cells in inflammatory breast cancer. Am. J. Surg. 214:666–671, 2017.
Fischbach, C., T. Spruss, B. Weiser, M. Neubauer, C. Becker, M. Hacker, A. Göpferich, and T. Blunk. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp. Cell Res. 300:54–64, 2004.
Gao, Y., X. Chen, Q. He, R. C. Gimple, Y. Liao, L. Wang, R. Wu, Q. Xie, J. N. Rich, K. Shen, and Z. Yuan. Adipocytes promote breast tumorigenesis through TAZ-dependent secretion of resistin. Proc. Natl. Acad. Sci. USA 117:33295–33304, 2020.
Grasset, E. M., M. Dunworth, G. Sharma, M. Loth, J. Tandurella, A. Cimino-Mathews, M. Gentz, S. Bracht, M. Haynes, E. J. Fertig, and A. J. Ewald. Triple-negative breast cancer metastasis involves complex epithelial-mesenchymal transition dynamics and requires vimentin. Sci. Transl. Med. 14:eabn7571, 2022.
Hammel, J. H., and E. Bellas. Endothelial cell crosstalk improves browning but hinders white adipocyte maturation in 3D engineered adipose tissue. Integr. Biol. 12:81–89, 2020.
Hapach, L. A., S. P. Carey, S. C. Schwager, P. V. Taufalele, W. Wang, J. A. Mosier, N. Ortiz-Otero, T. J. McArdle, Z. E. Goldblatt, M. C. Lampi, F. Bordeleau, J. R. Marshall, I. M. Richardson, J. Li, M. R. King, and C. A. Reinhart-King. Phenotypic heterogeneity and metastasis of breast cancer cells. Cancer Res. 81:3649–3663, 2021.
Harney, A. S., E. N. Arwert, D. Entenberg, Y. Wang, P. Guo, B. Z. Qian, M. H. Oktay, J. W. Pollard, J. G. Jones, and J. S. Condeelis. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 5:932–943, 2015.
Hildreth, A. D., F. Ma, Y. Y. Wong, R. Sun, M. Pellegrini, and T. E. O’Sullivan. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat. Immunol. 22:639–653, 2021.
Hodson, L., C. M. Skeaff, and B. A. Fielding. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 47:348–380, 2008.
Hotamisligil, G. S., N. S. Shargill, and B. M. Spiegelman. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91, 1993.
Hsiao, A. Y., T. Okitsu, H. Teramae, and S. Takeuchi. 3D tissue formation of unilocular adipocytes in hydrogel microfibers. Adv. Healthc. Mater. 5:548–556, 2016.
Ioannidou, A., S. Alatar, R. Schipper, F. Baganha, M. Åhlander, A. Hornell, R. M. Fisher, and C. E. Hagberg. Hypertrophied human adipocyte spheroids as in vitro model of weight gain and adipose tissue dysfunction. J. Physiol. 600:869–883, 2022.
Jackson, G. W., and D. F. James. The permeability of fibrous porous media. Can. J. Chem. Eng. 64:364–374, 1986.
Jernås, M., J. Palming, K. Sjöholm, E. Jennische, P. A. Svensson, B. G. Gabrielsson, M. Levin, A. Sjögren, M. Rudemo, T. C. Lystig, B. Carlsson, L. M. Carlsson, and M. Lönn. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 20:E832–E839, 2006.
Kitsis, R. N., and L. A. Leinwand. Discordance between gene regulation in vitro and in vivo. Gene Exp. 2:313–318, 1992.
Kleinert, M., C. Clemmensen, S. M. Hofmann, M. C. Moore, S. Renner, S. C. Woods, P. Huypens, J. Beckers, M. H. de Angelis, A. Schürmann, M. Bakhti, M. Klingenspor, M. Heiman, A. D. Cherrington, M. Ristow, H. Lickert, E. Wolf, P. J. Havel, T. D. Müller, and M. H. Tschöp. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 14:140–162, 2018.
Lee, M. J., and S. K. Fried. Optimal protocol for the differentiation and metabolic analysis of human adipose stromal cells. Methods Enzymol. 538:49–65, 2014.
Li, X., J. Xia, C. T. Nicolescu, M. W. Massidda, T. J. Ryan, and J. Tien. Engineering of microscale vascularized fat that responds to perfusion with lipoactive hormones. Biofabrication 11:014101, 2019.
Ling, L., J. A. Mulligan, Y. Ouyang, A. A. Shimpi, R. M. Williams, G. F. Beeghly, B. D. Hopkins, J. A. Spector, S. G. Adie, and C. Fischbach. Obesity-associated adipose stromal cells promote breast cancer invasion through direct cell contact and ECM remodeling. Adv. Funct. Mater. 30:1910650, 2020.
Lohmann, A. E., S. V. Soldera, I. Pimentel, D. Ribnikar, M. Ennis, E. Amir, and P. J. Goodwin. Association of obesity with breast cancer outcome in relation to cancer subtypes: a meta-analysis. J. Natl. Cancer Inst. 113:1465–1475, 2021.
Lumeng, C. N., J. L. Bodzin, and A. R. Saltiel. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 117:175–184, 2007.
Martin, A. D., M. Z. Daniel, D. T. Drinkwater, and J. P. Clarys. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int. J. Obes. Relat. Metab. Disord. 18:79–83, 1994.
Mazzarella, L., D. Disalvatore, V. Bagnardi, N. Rotmensz, D. Galbiati, S. Caputo, G. Curigliano, and P. G. Pelicci. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur. J. Cancer 49:3588–3597, 2013.
McDowell, S. A. C., R. B. E. Luo, A. Arabzadeh, S. Doré, N. C. Bennett, V. Breton, E. Karimi, M. Rezanejad, R. R. Yang, K. D. Lach, M. S. M. Issac, B. Samborska, L. J. M. Perus, D. Moldoveanu, Y. Wei, B. Fiset, R. F. Rayes, I. R. Watson, L. Kazak, M. C. Guiot, P. O. Fiset, J. D. Spicer, A. J. Dannenberg, L. A. Walsh, and D. F. Quail. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat. Cancer 2:545–562, 2021.
Mehta, A., A. M. Oeser, and M. G. Carlson. Rapid quantitation of free fatty acids in human plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 719:9–23, 1998.
Padmanaban, V., I. Krol, Y. Suhail, B. M. Szczerba, N. Aceto, J. S. Bader, and A. J. Ewald. E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573:439–444, 2019.
Pierobon, M., and C. L. Frankenfeld. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res. Treat. 137:307–314, 2013.
Piotrowski-Daspit, A. S., A. K. Simi, M. F. Pang, J. Tien, and C. M. Nelson. A 3D culture model to study how fluid pressure and flow affect the behavior of aggregates of epithelial cells. Methods Mol. Biol. 1501:245–257, 2017.
Quail, D. F., and A. J. Dannenberg. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15:139–154, 2019.
Rabhi, N., K. Desevin, A. C. Belkina, A. Tilston-Lunel, X. Varelas, M. D. Layne, and S. R. Farmer. Obesity-induced senescent macrophages activate a fibrotic transcriptional program in adipocyte progenitors. Life Sci. Alliance 5:e202101286, 2022.
Salans, L. B., S. W. Cushman, and R. E. Weismann. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J. Clin. Investig. 52:929–941, 1973.
Seo, B. R., P. Bhardwaj, S. Choi, J. Gonzalez, R. C. Andresen Eguiluz, K. Wang, S. Mohanan, P. G. Morris, B. Du, X. K. Zhou, L. T. Vahdat, A. Verma, O. Elemento, C. A. Hudis, R. M. Williams, D. Gourdon, A. J. Dannenberg, and C. Fischbach. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl. Med. 7:301ra130, 2015.
Shen, J. X., M. Couchet, J. Dufau, T. de Castro Barbosa, M. H. Ulbrich, M. Helmstädter, A. M. Kemas, R. Zandi Shafagh, M. A. Marques, J. B. Hansen, N. Mejhert, D. Langin, M. Rydén, and V. M. Lauschke. 3D adipose tissue culture links the organotypic microenvironment to improved adipogenesis. Adv. Sci. 8:e2100106, 2021.
Shi, Y., G. Zhang, Y. Wang, C. Ren, L. Wen, W. Zhu, X. Chen, and N. Liao. Presence of circulating tumor cells is associated with metabolic-related variables in postoperative patients with early-stage breast cancer. Chin. J. Cancer Res. 30:340–350, 2018.
Soukas, A., N. D. Socci, B. D. Saatkamp, S. Novelli, and J. M. Friedman. Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J. Biol. Chem. 276:34167–34174, 2001.
Tien, J., J. G. Truslow, and C. M. Nelson. Modulation of invasive phenotype by interstitial pressure-driven convection in aggregates of human breast cancer cells. PLoS ONE. 7:e45191, 2012.
Tien, J., Y. W. Dance, U. Ghani, A. J. Seibel, and C. M. Nelson. Interstitial hypertension suppresses escape of human breast tumor cells via convection of interstitial fluid. Cell. Mol. Bioeng. 14:147–159, 2021.
Tiwari, P., A. Blank, C. Cui, K. Q. Schoenfelt, G. Zhou, Y. Xu, G. Khramtsova, F. Olopade, A. M. Shah, S. A. Khan, M. R. Rosner, and L. Becker. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J. Exp. Med. 216:1345–1358, 2019.
Verboven, K., K. Wouters, K. Gaens, D. Hansen, M. Bijnen, S. Wetzels, C. D. Stehouwer, G. H. Goossens, C. G. Schalkwijk, E. E. Blaak, and J. W. Jocken. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci. Rep. 8:4677, 2018.
Visser, T. D., J. L. Oud, and G. J. Brakenhoff. Refractive index and axial distance measurements in 3-D microscopy. Optik 90:17–19, 1992.
Wang, Y. Y., C. Attané, D. Milhas, B. Dirat, S. Dauvillier, A. Guerard, J. Gilhodes, I. Lazar, N. Alet, V. Laurent, S. Le Gonidec, D. Biard, C. Hervé, F. Bost, G. S. Ren, F. Bono, G. Escourrou, M. Prentki, L. Nieto, P. Valet, and C. Muller. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2:e87489, 2017.
Weisberg, S. P., D. McCann, M. Desai, M. Rosenbaum, R. L. Leibel, and A. W. Ferrante. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 112:1796–1808, 2003.
Wueest, S., R. A. Rapold, J. M. Rytka, E. J. Schoenle, and D. Konrad. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia 52:541–546, 2009.
Yang, F., A. Carmona, K. Stojkova, E. I. Garcia Huitron, A. Goddi, A. Bhushan, R. N. Cohen, and E. M. Brey. A 3D human adipose tissue model within a microfluidic device. Lab Chip 21:435–446, 2021.
Acknowledgments
This work was supported by award U01 CA214292 from the National Cancer Institute and by award P30 DK046200 (Boston Nutrition Obesity Research Center; BNORC) from the National Institute of Diabetes and Digestive and Kidney Diseases. Y.W.D. was funded through the CURE Diversity Research Supplements Program at the National Cancer Institute, and M.C.O. was funded through the Undergraduate Research Opportunities Program at Boston University. Human breast-derived ASCs were provided by the Mayo Clinic Comprehensive Cancer Center and BNORC. We thank Miguel L. Batista, Jr. and Nabil Rabhi for insightful discussions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Yoseph W. Dance, Mackenzie C. Obenreder, Alex J. Seibel, Tova Meshulam, Joshua W. Ogony, Nikhil Lahiri, Laura Pacheco-Spann, Derek C. Radisky, Matthew D. Layne, Stephen R. Farmer, Celeste M. Nelson, and Joe Tien declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dance, Y.W., Obenreder, M.C., Seibel, A.J. et al. Adipose Cells Induce Escape from an Engineered Human Breast Microtumor Independently of their Obesity Status. Cel. Mol. Bioeng. 16, 23–39 (2023). https://doi.org/10.1007/s12195-022-00750-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-022-00750-y