Abstract
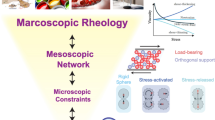
The relationship between microstructure changes and rheological properties in suspensions containing boehmite particles, which are well applied in various industrial wash-coating processes, was investigated by changing pH condition. The boehmite particles in suspensions were either well dispersed or aggregated depending on the pH, owing to the relative contributions of repulsive interaction between particles as well as hydrolysis and condensation reactions. Four groups of boehmite suspensions were classified as very low, intermediate, almost zero charge, and high pH regimes based on their colloidal behaviors, and their microstructural differences were investigated using transmission electron microscopy (TEM) and multi-speckle diffusing wave spectroscopy (MSDWS). For gel-like suspensions of three groups, various rheological properties such as shear viscosity, viscoelastic modulus, yield stress, and recovery behavior were extensively compared, and the results clearly demonstrated that a suspension with high yield stress was not fully recovered into the original state when disturbed at high shear rates.






Similar content being viewed by others
Data availability
Data will be made available on request.
References
Zheng Y, Song J, Xu X, He M, Wang Q, Yan L (2014) Peptization mechanism of boehmite and its effect on the preparation of a fluid catalytic cracking catalyst. Ind Eng Chem Res 53(24):10029–10034
Buining PA, Pathmamanoharan C, Jansen JBH, Lekkerkerker HNW (1991) Preparation of colloidal boehmite needles by hydrothermal treatment of aluminum alkoxide precursors. J Am Ceram Soc 74(6):1303–1307
Okubo T, Watanabe M, Kusakabe K, Morooka S (1990) Preparation of γ-alumina thin membrane by sol-gel processing and its characterization by gas permeation. J Mater Sci 25(11):4822–4827
Ghorbani-Choghamarani A, Tahmasbi B, Arghand F, Faryadi S (2015) Nichel Schiff-base complexes immobilized on boehmite nanoparticles and their application in the oxidation of sulfides and oxidative coupling of thiols as novel and reusable nano organometal catalysts. RSC Adv 5(112):92174–92183
Vatanpour V, Madaenia SS, Rajabi L, Zinadini S, Derakhshan AA (2012) Boehmite nanoparticles as a new nanofiller for preparation of antifouling mixed matrix membranes. J Membr Sci 401:132–143
Agrafiotis C, Tsetsekou A (2000) The effect of powder characteristics on washcoat quality. Part I: alumina washcoats. J Eur Ceram Soc 20(7):815–824
Knözinger H, Ratnasamy P (1978) Catalytic aluminas: Surface models and characterization of surface sites. Catal Rev Sci Eng 17(1):31–70
Yang CY, Shih WY, Shih WH (2001) Effects of boehmite-coating thickness on the consolidation and rheological properties of boehmite-coated SiC suspensions. J Am Ceram Soc 84(12):2834–2840
Buining PA, Philipse AP, Lekkerkerker HNW (1994) Phase behavior of aqueous dispersions of colloidal boehmite rods. Langmuir 10(7):2106–2114
Davis JA, James RO, Leckie JO (1978) Surface ionization and complexation at the oxide/water interface: i. Computation of electrical double layer properties in simple electrolytes. J Colloid Interf Sci 63(3):480–499
Chun MS, Lee S, Lee TS, Cho JS (2004) Microstructure and shear modulus in concentrated dispersions of bidisperse charged spherical colloids. Korea-Aust Rheol J 16(1):17–26
Chun B, Chun MS (2021) Electrostatic potential analysis in polyelectrolyte brush-grafted microchannels filled with polyelectrolyte dispersion. Micromachines 12(12):1475
Karouia FK, Boualleg M, Digne M, Alphonse P (2013) Control of the textural properties of nanocrystalline boehmite (γ-AlOOH) regarding its peptization ability. Powder Tech 237:602–609
Chen XY, Lee SW (2007) pH-Dependent formation of boehmite (γ-AlOOH) nanorods and nanoflakes. Chem Phys Lett 438(4–6):279–284
Huang W, Liu G, Qi T, Li X, Zhou Q, Peng Z (2020) Effects of pH and ions on the morphological evolution of boehmite prepared by hydrothermal treatment of ultrafine Bayer gibbsite. CrystEngComm 22(42):6983–6992
Pierre AC, Pajonk GM (2002) Chemistry of aerogels and their applications. Chem Rev 102(11):4243–4266
Cushing BL, Kolesnichenko VL, O’Connor CJ (2004) Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem Rev 104(9):3893–3946
Danks AE, Hall SR, Schnepp Z (2016) The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater Horiz 3(2):91–112
Makeki H, Durães L, Portugal A (2014) An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J Non-Cryst Solid 385:55–74
Linhares T, de Amorim MTP, Durães L (2019) Silica aerogel composites with embedded fibres: a review on their preparation, properties and applications. J Mater Chem A 7(40):22768–22802
Anovitz LM, Zhang X, Soltis J, Nakouzi E, Krzysko AJ, Chun J, Schenter GK, Graham TR, Rosso KM, De Yoreo JJ, Stack AG, Bleuel M, Gagnon C, Mildner DFR, Ilavsky J, Kuzmenko I (2018) Effects of ionic strength, salt, and ph on aggregation of boehmite nanocrystals: Tumbler small-angle neutron and X-ray scattering and imaging analysis. Langmuir 34(51):15839–15853
M’Barki A, Bocquet L, Stevenson A (2017) Linking rheology and printability for dense and strong ceramics by direct ink writing. Sci Rep 7(1):1–10
Sudreau I, Manneville S, Servel M, Divoux T (2022) Shear-induced memory effects in boehmite gels. J Rheol 66(1):91–104
Foundas M, Bricher LG, Fornasiero D, Morris GE (2015) Boehmite suspension behaviour upon adsorption of methacrylate–phosphonate copolymers. Powder Tech 269:385–391
Vishista K, Gnanam FD (2004) Role of deflocculants on the rheological properties of boehmite sol. Mater Lett 58(10):1576–1581
Weston JS, Chun J, Schenter G, Weigandt K, Zhang M, Rosso KM, Anovitz LM (2020) Connecting particle interactions to agglomerate morphology and rheology of boehmite nanocrystal suspensions. J Colloid Interf Sci 572:328–339
Park BS, Jung KI, Lee SJ, Lee KY, Jung HW (2018) Effect of particle shape on drying dynamics in suspension drops using multi-speckle diffusing wave spectroscopy. Colloid Polym Sci 296(5):971–979
Jung KI, Park BS, Lee SJ, Noh SM, Jung HW (2020) Effect of immiscible secondary fluid on particle dynamics and coffee ring characteristics during suspension drying. Materials 13(15):3438
Lee JY, Hwang JW, Jung HW, Kim SH, Lee SJ, Yoon K, Weitz D (2013) Fast dynamics and relaxation of colloidal drops during the drying process using multi-speckle diffusing wave spectroscopy. Langmuir 29(3):861–866
Walls HJ, Caines SB, Sanchez AM, Khan SA (2003) Yield stress and wall slip phenomena in colloidal silica gels. J Rheol 47(4):847–868
Weston JS, Harwell JH, Grady BP (2017) Rheological characterization of yield stress gels formed via electrostatic heteroaggregation of metal oxide nanoparticles. Soft Matter 13(38):6743–6755
Lin YJ, Chuang WT, Hsu S (2019) Gelation mechanism and structural dynamics of chitosan self-healing hydrogels by in Situ SAXS and coherent X-ray scattering. ACS Macro Lett 8(11):1449–1455
Sugioka Y, Nakamura J, Ohtsuki C, Sugawara-Narutaki A (2021) Thixotropic hydrogels composed of self-assembled nanofibers of double-hydrophobic elastin-like block polypeptides. Int J Mol Sci 22(8):4104
Kosmulski M (2020) The pH dependent surface charging and points of zero charge. VIII Update Adv Colloid Interface Sci 275:102064
Bender J, Wagner NJ (1996) Reversible shear thickening in monodisperse and bidisperse colloidal dispersions. J Rheol 40(5):899–916
Boutenel F, Dusserre G, Aimable A, Chartier T, Cutard T (2021) Rheophysical study of dispersed alumina suspensions. Powder Tech 393:630–638
Gürgen S, Li W, Kuşhan MC (2016) The rheology of shear thickening fluids with various ceramic particle additives. Mater Des 104:312–319
Alaee P, Kamkar M, Arjmand M (2022) Fumed silica-based suspensions for shear thickening applications: a full-scale rheological study. Langmuir 38(16):5006–5019
Cetinel AF, Simon RA (2012) Nanoparticle assisted coagulation of aqueous alumina suspensions. Mater Res 15:81–89
Kowalska M, Krztoń-Maziopa A, Babut M, Mitrosz P (2020) Rheological and physical analysis of oil-water emulsion based on enzymatic structured fat. Rheol Acta 59(10):717–726
Acknowledgements
This study was supported by the Ministry of Trade, Industry, and Energy (MOTIE, Korea) under the Industrial Technology Innovation Program (No. 20011712) and by National Research Foundation of Korea (NRF) grants funded by the Ministry of Science and ICT (MSIT) of the Korean government (No. NRF-2016R1A5A1009592 and NRF-2021M3H4A6A01041234).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, G.W., Kim, S.H., Lee, D.Y. et al. Effect of solution pH on the microstructural and rheological properties in boehmite suspensions. Korea-Aust. Rheol. J. 35, 1–10 (2023). https://doi.org/10.1007/s13367-022-00046-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13367-022-00046-7