Abstract
Human cytomegalovirus (CMV) is a widespread persistent herpes virus requiring lifelong immune surveillance to maintain latency. Such long-term interactions with the immune system may be associated with deleterious effects including immune exhaustion and senescence. Regarding the COVID-19 pandemic, we asked whether CMV-specific cellular and humoral activity could influence immune responses toward SARS-CoV-2 and/or disease severity. All adults with mild (n = 15) and severe (n = 14) COVID-19 were seropositive for anti-CMV IgG, but negative for IgM antibodies. Antibody titers did not correlate with COVID-19 severity. Six patients presented elevated frequencies of CMV-specific CD4 + and CD8 + T cells producing IFNγ, IL-17, and TNFα, designated as CMV high responders (hiT CMV). In comparison to low CMV responders, hiT CMV individuals exhibited higher frequencies of SARS-CoV-2-specific CD4 + IL-17 + and CD8 + IFNγ + , IL-17 + or TNFα + T cells. These results indicate that high frequencies of CMV-specific T cells may be associated with a SARS-CoV-2-reactive profile skewed toward Th17-dominated immunity.
Graphical abstract

Similar content being viewed by others
Introduction
Human cytomegalovirus (CMV) belongs to the Herpesviridae family causing persistent latent infection in immunocompetent individuals. After primary infection, the host may experience several episodes of reinfection or reactivation, as the virus persists in a latent phase in hematopoietic progenitors and cells within the myeloid lineage [1]. Cytomegalovirus represents one of the most common infections globally, with a prevalence ranging from 50 to 95% depending on age and socioeconomic status [2]. Studies from Southern Brazil [3,4,5] show an 88–98% IgG prevalence in adults below 55 years old, and 100% in older individuals, with anti-CMV IgM present in 2.4% of the adult population, most of them under 35 years old and probably representing new infections [5].
The chronic interaction of CMV with the immune system, necessary for maintaining latency and preventing pathology, may eventually be associated with deleterious effects reflecting immune exhaustion and senescence, especially in the elderly [6,7,8], while in CMV-positive young people and adults, the presence of highly differentiated and sensitized T cells is potentially an immunological advantage for responses against other pathogens [9,10,11,12]. We asked whether the severity of SARS-CoV-2 disease, which has pathological mechanisms associated with severe acute respiratory syndrome that include dysfunctional and exacerbated immune responses [13], was associated with the immune response to CMV infection. We found that, although anti-CMV IgG titers did not correlate with COVID-19 severity, high frequencies of CMV-specific CD4 + and CD8 + T cells were associated with elevated levels of SARS-CoV-2-specific IL-17-producing CD4 + and CD8 + T cells. This suggests that CMV may skew the anti-SARS-CoV-2 activity toward an immune phenotype polarized to a Th17 type of response which could influence inflammation and the course of disease.
Methods
Patients
This is a case–control study focused on the analysis of CMV humoral and cellular immune responses in mild and severe COVID-19 patients. A convenience sample of COVID-19 patients was obtained from Moinhos de Vento and Restinga Extremo Sul hospitals, located in Porto Alegre, Brazil. Adults with confirmed SARS-CoV-2 positivity that arrived at either hospital were invited to participate in the study. Participants included were recruited from June to December 2020, all > 18 years of age who presented with cough and/or axillary temperature above 37.8 °C and/or sore throat, and who were hospitalized, in emergency rooms or outside health units. Nasopharyngeal and oropharyngeal swabs were collected to confirm the COVID-19 diagnosis. Clinical and demographic data were collected at inclusion. Disease severity was obtained according to the World Health Organization classification following a standardized protocol [14]: severe COVID-19 patients were classified based on oxygen saturation < 90% in room air, with signs of severe respiratory distress and pneumonia. Mild disease was defined by the absence of any of the criteria above for severe COVID-19. The study was conducted according to the Declaration of Helsinki. This study was approved by the Institutional Review Board (IRB 30,749,720.4.1001.5330) at Hospital Moinhos de Vento and Ethics Committee from Fundação Faculdade Federal de Ciências Médicas de Porto Alegre (CEP-UFCSPA) (CAAE 30,749,720.4.3001.5345). Informed consent was obtained from all participants or legal guardians.
SARS-CoV-2 RT-qPCR
A qualitative RT-PCR assay to SARS-CoV-2 was performed for all participants as detailed in our previous work [15]: bilateral nasopharyngeal and oropharyngeal swabs were collected and transported in medium with saline solution and RNAlater®, RNA Stabilization Solution (Catalog number AM7021, Invitrogen™). For RNA extraction, MagMax™ Viral/Pathogenic Nucleic Acid Isolation Kit (Applied Biosystems) was used in the KingFisher Duo Prime System (ThermoFisher, USA) automated platform. The RT-PCR assay was performed in a 10 µL total reaction, using Path™ 1-Step RT-qPCR Master Mix, CG (catalog number A15299, AppliedBiosystems), and TaqMan™ 2019-nCoV Assay Kit v1 (catalog number A47532, AppliedBiosystems). As control, 5 µL (200 copies/µL) of the TaqMan™ 2019-nCoV Control Kit v1 (catalog number A47533, AppliedBiosystems) was used. QuantStudio 5 (ThermoFisher Scientific, USA) was applied to perform the PCR.
ELISA
IgG and IgM antibodies for CMV and IgG and IgA for SARS-CoV-2 S-RBD (Spike-Receptor Binding Domain) and N (Nucleocapsid) peptides were analyzed. Heat-Inactivated (56 ºC for 60 min) plasma samples from the study participants were used in the assay.
Anti-CMV antibodies were tested using commercial kits provided by BioClin (Brazil). Qualitative IgM determination was performed by enzyme immunoassay (Biolisa CMV IgM, K123—BioClin). Positive results were defined by absorbance greater than the cut-off calibrator. For IgG, we performed a quantitative determination (Biolisa CMV IgG, K122—BioClin). To quantify the sample absorbances as IU/mL (International Units per mL), five reference standard solutions containing IgG anti-CMV antibodies in different concentrations were used in the assay, and a concentration curve was obtained from each absorbance and known IgG (in IU/mL). Samples’ absorbances were converted to IU/mL based on the assay concentration curve. Negative and positive controls from the kits were used in the assays to confirm test accuracy.
For anti-S-RBD and anti-N IgG and IgA, we followed our previous study protocol [15]. Briefly, ELISA plates (Kasvi, Brazil) were coated overnight with 1 μg/mL of SARS-CoV-2 Spike Protein (S-RBD; Catalog nr. RP-87678, Invitrogen) or N peptide (Fiocruz, Brazil). Next, blocking buffer was added for 1 h at room temperature. Samples were serially diluted in 0.05% PBS-Tween 20 (milk 1%) starting at 1:25 and incubated for 2 h at 37 °C. Secondary antibodies diluted in 0.05% PBS-Tween were then added and plates were incubated for 1 h at room temperature. Anti-human peroxidase produced in rabbits (Catalog nr. IC-1H01—Rhea Biotec, Brazil) was used for IgG, and anti-human peroxidase produced in goat (Catalog nr. A18781—Invitrogen, USA) was used for IgA, both at a 1:10,000 dilution. The assay was developed with TMB Elisa Substrate—High Sensitivity (Abcam, United Kingdom) for 30 min, and 1 M chloric acid was added to stop the reaction. An ELISA reader (Biochrom EZ 400) was used to measure anti-CMV and anti-SARS-CoV-2 antibodies with a 450 nm O.D. To calculate the area under the curve (AUC), a baseline of 0.07 was applied [16].
PBMC Isolation
Blood samples were collected in EDTA tubes and stored at room temperature. PBMCs were isolated by density gradient centrifugation, using Ficoll™ PLUS (GE Healthcare®). Cells were resuspended in Fetal Bovine Serum (FBS) with 5% DMSO and stored in liquid nitrogen until analysis.
T cell In vitro simulation
After thawing, PBMCs were counted and viability was evaluated, with subsequent plating in 96-well plates at 3 × 105 PBMCs per well in 100 μL of RPMI-1640 medium (Sigma-Aldrich—R8758) supplemented with fetal bovine serum (10%) and antibiotics (100 IU penicillin/mL, 100 μg streptomycin/mL and 2 mM L-glutamine (Lonza, Belgium)). Cells were stimulated with a CMV mega pool (2 µg/mL, composed of 42 peptides from cytomegalovirus, including pp50, pp65, IE1, IE2, and envelope glycoprotein B—Mabtech, Sweden) or 1 µg/mL of peptide PepTivator SARS-CoV-2 Prot S (130–126-700—Miltenyi Biotec, Germany), PepTivator SARS-CoV-2 Prot N (130–126-698—Miltenyi Biotec, Germany) or PepTivator SARS-CoV-2 Prot M (130–126-702—Miltenyi Biotec, Germany). PMA (50 ng/mL, Sigma, USA) plus ionomycin (1 μg/mL, Cayman chemical company, USA) and DMSO were used as positive and negative controls, respectively. Plates were incubated at 37 °C/5% CO2 for 18 h. Three hours before harvesting the cells, 1 μg/ml of Golgi Plug (BD Biosciences, USA) was added to each well. Cells were labeled and analyzed by flow cytometry to establish their phenotypes as follows.
CD4 + and CD8 + immunophenotyping
Cells were labeled with BD Horizon™ Fixable Viability Stain 510 for viability, and then with surface marker antibodies: anti-CD3-PE-Cy7 (clone SK7), anti-CD4-PerCP-Cy5.5 (clone RPA-T4), and anti-CD8-APC-H7 (clone SK1). For intracellular cytokine labeling, cells were first stained with surface markers, and then fixed, and permeabilized with Cytofix/Cytoperm kit (BD Biosciences-Pharmingen, USA). They were then stained with anti-IFNγ-FITC (clone 4S.B3), anti-TNFα-APC (clone MAb11), and anti-IL-17-PE (clone SCPL1362) antibodies. For surface antibody staining, incubation was for 30 min at room temperature in the dark. For intracellular antibodies, the interval was 40 min at 2–8 °C. Samples were analyzed using BD Biosciences—FACSCanto II and FlowJo 10.7.1 software. CMV- and SARS-CoV-2-specific CD4 + and CD8 + cells are represented as frequencies of viable PBMCs.
Serum cytokine measurements
IFN-γ, TNF, IL-4, IL-6, IL-10, and IL-17A concentrations in inactivated serum were analyzed with a commercial BD CBA Human Th1/Th2/Th17 cytokines kit (cat 560,484, BD Inc., USA), as described in our previous work [15]. Briefly, a mixture of capture beads coated with capture antibodies specific for each cytokine and a phycoerythrin (PE) detection reagent were used according to the manufacturer’s instruction. Then, samples were measured on the BD FACS Canto II flow cytometer and analyzed by FCAP Array software 3.0. Individual cytokine concentrations were indicated by their fluorescent intensities and represented in pg/mL.
Statistical analysis
The Shapiro Wilk test was used to determine normal distribution of results. For age, mean ± standard deviation was considered, and an unpaired T-test with Welch’s correction was applied. Fisher’s exact test was used to calculate proportions between groups. For anti-CMV IgG, days of symptoms until blood collection and CD4 + and CD8 + specific T cell frequencies, median ± interquartile range was considered. A nonparametric two-tailed Mann Whitney test was used for comparison between two groups and the Kruskal–Wallis test for comparisons between more than two groups. Spearman r was used for correlations between variables (corrplot package in R). Statistical analysis was conducted in GraphPad Prism 9.0 and R 4.1.
Results
As shown in Table 1, severe COVID-19 patients were significantly older than mild patients (53.6 ± 17.9 vs 38.8 ± 12.6, p < 0.05), in addition to having a higher proportion of comorbidities (9 (64.3%) vs 2 (13.3%), p < 0.001) such as hypertension, congenital heart disease, chronic obstructive pulmonary disease (COPD), Diabetes Mellitus 1 or 2, prior stroke, heart failure, and obesity. These results align with previous studies that attribute comorbidities as a risk factor for the severity of COVID-19 [17, 18].
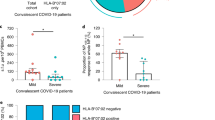
To investigate cytomegalovirus prevalence in mild and severe COVID-19 patients, we evaluated anti-CMV IgG and IgM positivity to determine the presence of primary infection (IgM +), latency (IgG +), or reactivation cases (IgM + , IgG +) in our sample. While none of the patients with confirmed COVID-19 was CMV IgM-seropositive (Fig. 1A), all were IgG-seropositive. No significant difference in IgG titers was found according to the severity of the disease (Fig. 1B).
Qualitative anti-CMV IgM (A) and quantitative anti-CMV IgG (B) assays in mild (n = 15) and severe (n = 14) COVID-19 patients. Samples of serum were diluted according to the kit instructions. Cut-offs of 0.194 (absorbance) for IgM and 1.2 (IU/mL) for IgG were calculated based on the calibrator of the commercial kits used for the analysis. (C) Comparison of anti-CMV IgG quartiles by age in severe COVID-19 patients (n = 14). Statistical analysis using nonparametric Mann–Whitney test for A–B and Kruskal–Wallis test for C, with p values < 0.05 (*), < 0.01 (**) and < 0.001 (***)
The wide range of anti-CMV IgG titers in severe COVID-19 patients (18.4 IU/mL (2.8–39.5)) might be attributable to age variation in this group (53.6 ± 17.9 years). Based on studies that showed associations between anti-CMV IgG titers and age [19], we separated severe patients by anti-CMV IgG quartiles and found that individuals with higher IgG titers indeed tended to be older (Fig. 1C). We also assessed the proportion of comorbidities in each anti-CMV IgG quartile and observed that six of the nine severe patients with comorbidities (66.7%) were concentrated in the third and fourth quartiles, whereas four of the five individuals with no comorbidities (80%) belonged to the first and second IgG quartiles. In contrast, neither age nor the presence of comorbidities correlated with anti-CMV IgG titers in patients with mild COVID-19.
We then asked if CMV-specific T responses were different in COVID-19 patients according to disease severity. We assessed the frequencies of CD4 + and CD8 + CMV peptide-specific T cells by the production of IFNγ, IL-17, and TNFα by flow cytometry (gating strategy presented in Fig. 2A). Despite the lack of difference in terms of severity of disease, a group of CMV responders [patients’ number 1 to 6 (Fig. 2B–G)], exhibited frequencies of CMV-specific cells above the third quartile for at least five of the six subpopulations analyzed (CD4 + IFNγ + > 1.1%; CD4 + IL17 + > 4.9%; CD4 + TNFα + > 1.83%; CD8 + IFNγ + > 1.75%; CD8 + IL-17 + > 1.57%; CD8 + TNFα + > 3.9%). Figure 3 shows that, overall, CMV-specific T responses had significant correlations with each other, in contrast to the absence of association with anti-CMV IgG titers.
A Gate strategy for T cell responses after stimulation with CMV peptides. The same gates were applied for SARS-CoV-2 peptides and controls. Each panel depicts how peptide-specific CD4 + and CD8 + T cells were selected: viable PBMCs were isolated through size and complexity (FSC-A, SSC-A) together with BD Horizon™ Fixable Viability Stain 510. Anti-CD3 stained cells were then selected to isolate only lymphocytes that were positive for anti-CD4 or anti-CD8 with the respective cytokines (IFNγ, IL-17 or TNFα). B–G Frequencies of CMV-specific CD4+ and CD8+ T cells in mild (n = 15) and severe (n = 14) COVID-19 patients. T responses represented in frequencies of parent: B CD4 + IFNγ + , (C) CD4 + IL-17 + , D CD4 + TNFα + , E CD8 + IFNγ + , F CD8 + IL-17 + , and G CD8 + TNFα + , after stimulation with CMV peptides. Bars represent median with interquartile range, and dots represent each patient. Six individuals from mild (1, 2, 3) and severe (4, 5, 6) groups exhibited frequency of cells above the third quartile for at least 5 of the 6 subpopulations analyzed. Statistical analysis by Mann–Whitney test, with p values < 0.05 (*), < 0.01 (**) and < 0.001 (***)
Correlation matrix between anti-CMV IgG titer and CMV-specific CD4 + and CD8 + T cell frequencies in COVID-19 patients (n = 29). Anti-CMV IgG titers presented in IU/mL and T responses in frequencies of parent. Circle size and color intensity indicate the strength of correlation by Spearman r (bigger and darker circles denote stronger associations), and colors depict positive (blue) or negative (red) correlations, with p < 0.05 (*), < 0.01 (**) and < 0.001 (***)
By separating this group of CMV high T responders (designated “hiT CMV”) from those with lower levels of response (“loT CMV”), we investigated SARS-CoV-2-specific CD4 + and CD8 + cells in both groups following stimulation of PBMCs with Spike (S), Membrane protein (M) or Nucleocapsid (N) peptides. We matched baseline characteristics between hiT and loT CMV groups for further analysis. Mean age of the hiT CMV group (n = 6) was 46 ± 9.4 years, similar to 48 ± 19.2 years of the loT CMV group (n = 10). Three patients (50%) in the hiT group and 5 (50%) in the loT CMV group presented with one or more comorbidities. The number of symptomatic days prior to blood collection was 13 (10.5–20.2) for the hiT patients and 10.5 (3.5–16.2) for the loT group.
hiT CMV individuals showed an increase in the frequencies of SARS-CoV-2-specific CD4 + IL-17 + , CD8 + IL-17 + , CD8 + TNFα + , and CD8 + IFNγ + cells (Fig. 4B–G), whereas loT CMV showed elevated CD4 + TNFα + frequencies for all three peptides analyzed (Supplementary Table 1). A comparison of the proportions of SARS-CoV-2-specific cytokine production between hiT and loT CMV individuals revealed that S- and N-specific CD4 + and CD8 + profiles were skewed to a Th17 type response in the hiT CMV group, with predominant IL-17-producing cells and an absence of CD4 + TNFα + cells (Fig. 4H). PBMCs stimulated with M peptide showed a less skewed response profile in the hiT CMV group. Thus, unlike the polarized Th17 profile stimulated by S and N peptides in hiT CMV responders, an overall more diversified phenotype of response was observed in the loT CMV individuals.
A Gating representation showing SARS-CoV-2-specific CD4 + and CD8 + frequencies in hiT and loT CMV individuals. B–G SARS-CoV-2-specific responses after stimulation with Spike B–C, Membrane D–E or Nucleocapsid F–G peptides, respectively, in hiT CMV (n = 6) and loT CMV (n = 10) COVID-19 patients. T responses represented in frequencies of parent. Bars represent the median and interquartile range, and dots represent each individual, with white and black circles representing mild and severe COVID-19 patients, respectively. H Stacked histogram of SARS-CoV-2-specific CD4 + and CD8 + T cells in hiT and loT CMV groups after stimulation with Spike, Membrane or Nucleocapsid peptide pools. Each fraction refers to the median frequency of IFNγ, IL-17 or TNFα-producing T cells upon stimulation. Statistical analysis using the nonparametric Mann–Whitney test, with p values < 0.05 (*), < 0.01 (**) and < 0.001 (***)
We then investigated possible associations between responses against the two viruses in CMV high and low T responders. Figure 5A–B shows that anti-CMV IgG correlated positively with anti-RBD IgG and anti-N IgA antibodies in hiT CMV, but not loT CMV individuals. In the hiT CMV group, anti-RBD IgG levels, but not IgA, correlated positively with all other anti-SARS-CoV-2 antibodies assayed. Anti-S-RBD and anti-N IgG were positively correlated with anti-N IgA in the loT CMV group.
Correlation matrices between CMV- and SARS-CoV-2-specific responses. A–B anti-CMV IgG and anti-SARS-CoV-2 (RBD or N-specific) IgG and IgA correlation matrix in (A) hiT CMV (n = 6) and B loT CMV (n = 10) groups. C–D Correlation between CMV- and SARS-CoV-2-specific T responses in C hiT CMV (n = 6), and D loT CMV (n = 10) groups. CD4 + and CD8 + cells represented in frequencies of viable PBMCs. Circle size and color intensity indicate the strength of correlation by Spearman r (bigger and darker circles denote stronger associations), and colors exhibit positive (blue) or negative (red) correlations, with p < 0.05 (*), < 0.01 (**) and < 0.001 (***)
Next, we asked if the frequency of CMV-specific T cells producing IFNγ, IL-17, or TNFα correlated with SARS-CoV-2-specific responses. Figure 5C shows that, in the hiT CMV group, both CD8 + and CD4 + IFNγ + responses to CMV positively correlated with anti-SARS-CoV-2 IFNγ + responses, while only CMV-specific CD4 + IL-17 + consistently correlated positively with IL-17 + anti-SARS-CoV-2 responses; anti-CMV CD8 + IL-17 + , instead, negatively correlated with IL-17 + anti-SARS-CoV-2 responses. Correlations of CMV-specific CD4 + TNFα + cells were also mostly positive between them and anti-SARS-CoV-2 TNFα + , except for CD8 + TNFα + cells and anti-M TNFα + cells (both CD4 + and CD8 + , Fig. 5E). For the loT CMV group, these patterns were not observed (Fig. 5D).
Finally, considering the elevated frequency of IL-17 responses in the hiT CMV individuals, we analyzed Th1 (IFNγ, TNFα), Th2 (IL-4, IL-10) and Th17 (IL-6, IL-17) cytokines in the patients’ serum to investigate if there were systemic implications related to the Th17 skewed profile observed. As shown in Fig. 6A–F, we found no differences in the concentration of these cytokines between hiT and loT CMV groups. However, their profile, or proportion, was unique for each group: hiT CMV individuals exhibited more IL-17 and IL-6 than any other cytokine, whereas the loT CMV group presented an IL-6 and IL-10 enriched state (Fig. 6G). This distinct IL-17 predominance corroborates our findings regarding the peptide-specific T cell responses with a Th17 polarized profile in the hiT CMV group.
Serum Th1, Th2, and Th17 cytokines in hiT (n = 6) and loT CMV (n = 10) groups: A IFNγ, B IL-4, C IL-6, D IL-10, E IL-17A and F TNFα. Values represented in pg/mL. Bars represent the median and interquartile range, and dots represent each individual, with white and black circles representing mild and severe COVID-19 patients, respectively. G Proportion of serum cytokines in hiT and loT CMV groups. Values refer to the median concentration (pg/mL) of each cytokine in hiT and loT CMV groups and are normalized to 100% of all cytokines. Statistical analysis using the nonparametric Mann–Whitney test, with p values < 0.05 (*), < 0.01 (**) and < 0.001 (***). H–I Matrix correlation of serum cytokines in (H) hiT and (I) loT CMV groups. Circle size and color intensity indicate the strength of correlation by Spearman r (bigger and darker circles denote stronger associations), and colors depict positive (blue) or negative (red) correlations, with p values < 0.05 (*), < 0.01 (**) and < 0.001 (***)
The balance of Th1/Th2/Th17 serum cytokines was analyzed through a matrix correlation in each group. Figure 6H–I shows that the loT CMV group exhibited strong positive correlations between Th1, Th2 and Th17 cytokines, suggesting a balance between pro and anti-inflammatory responses during COVID-19. On the other hand, hiT CMV individuals did not show the same equilibrated profile, with negative correlations between Th2 (IL-4) with Th1 (IFNγ and TNFα) cytokines.
Altogether, these data indicate that the predominant CD4 + IL-17 + frequencies toward CMV and SARS-CoV-2 may have systemic implications, including a higher proportion of serum IL-17A compared to other cytokines, and an imbalance of Th1, Th2 and Th17 immune profiles during infection.
Discussion
Since the SARS-CoV-2 outbreak, countless efforts have been made to investigate and understand risk factors related to a worse COVID-19 prognosis. The phenomena of cytomegalovirus reactivation in COVID-19 patients, in addition to longer ICU stay and lower chances of convalescence [20,21,22,23,24,25,26], highlight how important it is to study the impact of CMV infection and latency in the immune defense against SARS-CoV-2.
Given the mean age of our sample ranging from 20 to 81 years (45.9 ± 16.8 years), the absolute positivity rate for IgG showed the expected CMV prevalence in Southern Brazil [3,4,5]. We found that, despite the high prevalence of anti-CMV IgG, which indicates previous infection and latency (IgG + and IgM-), antibody titers did not correlate with severity of COVID-19 or humoral responses to SARS-CoV-2. Additionally, age and comorbidities did not significantly correlate with anti-CMV IgG titers in severe COVID-19 patients. This was perhaps unexpected given published studies showing associations between high titers of IgG to cytomegalovirus with disease progression and mortality in COPD [27, 28], hypertension [29], and cardiovascular diseases [30], which have all been identified as risk factors for COVID-19 severity [31].
We also found that CMV-specific T cell responses were not associated with COVID-19 clinical course. Strikingly, in the individuals with higher CMV-specific T responses, CD4 + and CD8 + immune profiles following Spike and Nucleocapsid stimulation in vitro were polarized toward a Th17-type of response, in contrast to more heterogeneous proportions of Th1 and Th17 cytokines in loT CMV individuals. Immune responses orchestrated by IL-17 are commonly associated with defense against extracellular fungi and bacteria through the recruitment of neutrophils and mononuclear phagocyte activation [32, 33]. Th17-dominated immunity is not the most effective type of response against viruses, which is associated with type 1 immunity and IFNγ production [34]. This agrees with recent studies that show IL-17 as a dysfunctional type of response against SARS-CoV-2, responsible for so-called “immune misfiring” [35], and associated with a worse prognosis of the disease, including mortality [36, 37]. Nevertheless, we could not confirm a relationship between the Th17 phenotype displayed by hiT CMV individuals and disease severity. It is possible that the “immune misfiring” is not as obvious as death or hospitalization—in the sense that infection seems to leave long-lasting symptoms, often neurological—and thus worth following up. In this context, the Th17 immune profile uncovered in this study, in association with CMV infection, might be implicated in the disease’s recovery process and incidence of inflammatory injury.
The mechanism responsible for the Th17 unbalanced response during COVID-19 is unclear. Hasan et al. [38] suggested that in some individuals, SARS-CoV-2 infection initiates a predominant IL-17-enriched chemokine transcriptional response while producing a low to moderate antiviral response by impairing interferon regulatory factors. The elevated frequency of SARS-CoV-2-specific CD4 + IL-17 + cells, in addition to an absence of CD4 + TNFα + response in the hiT CMV group, also points to a polarized T helper cell profile with predominant Th17-mediated activity. In line with this, Mason and colleagues [50] investigated the CMV secretome during latency and found an elevated production of chemokine ligand (CCL)8, responsible for the recruitment of CD4 + T cells, and, more importantly, a downregulation of CMV-specific IFNγ + and TNFα + CD4 + T cells, proposing it as a virus strategy to inhibit antiviral effector functions of recruited T cells and to skew it to a more favorable condition, as observed in our study in the hiT CMV individuals.
Also, because the development of Th17 cells during viral infections correlates with high levels of IL-6 and transforming growth factor-β (TGFβ) [39, 40], the inflammatory environment created by SARS-CoV-2 infection, marked by IL-1β, IL-17A, IL-18, and IL-6 upregulation [41], might be a potential factor involved in the unbalanced immune profile uncovered in the present study. The excess of SARS-CoV-2-specific CD8 + IFNγ, TNFα, and IL-17-producing cells also highlight the exacerbated inflammatory state evidenced in CMV high responders. In fact, a recent study by Naendrup and colleagues [42] found that most COVID-19 patients with cytomegalovirus or EBV reactivations were under systemic corticosteroid treatment due to a hyper inflammation state.
How anti-CMV T response could contribute or be related to this process could not be confirmed in this study. Besides, most existing research in this area focuses only on CMV seropositivity and its impact on T cell senescence and disease severity [43, 44]. Jo et al. [44] found that putatively senescent SARS-CoV-2-reactive CD8 + T cell populations were higher in CMV-seropositive young individuals than in seronegative ones, whereas Shrock et al. [43] observed that CMV seropositivity correlated with the hospitalization rate among COVID-19 patients. Our results suggest that CMV-specific T responses can also be investigated as a marker of immune polarization during SARS-CoV-2 infection.
Could pre-existing CMV-specific T cells be enhancing pro-inflammatory immunity mounted against SARS-CoV-2? Furman et al. [45] found that, upon influenza vaccination, CMV-seropositive young adults presented an upregulated immune function marked by elevated levels of IFNγ and increased CD8 + T cell sensitivity compared to CMV-seronegative individuals. Also, Barton et al. [46] showed in mice that CMV infection can protect animals against lethal infection with certain other pathogens by a mechanism involving prolonged production of cytokines and systemic activation of macrophages, thereby upregulating the basal activation state of innate immunity against subsequent infections. Moreover, some studies suggest that viral latency caused by Epstein-Barr (EBV) and CMV are responsible for a pro-inflammatory polarization that reduces Th2 upregulation and therefore decreases the incidence of allergies [47]. Thus, CMV latency may modulate immune responses to other antigens through a polarized cytokine environment. Another possibility is that CMV-specific T cells could be just contributing to an already dysfunctional immune response against SARS-CoV-2. Limaye and Boeckh [48] imply that, in the context of other respiratory infections, cytomegalovirus can prolong a pre-existing inflammatory environment through the upregulation of cytokines and inflammatory mediators. Finally, CMV-specific T cells could be cross-reacting with SARS-CoV-2 epitopes and leading to the augmentation of pro-inflammatory responses during infection. A preprint of research from Weber et al. [49] detected T cell cross-reactivity to cytomegalovirus and SARS-CoV-2 epitopes, partially explaining why individuals with a high frequency of CMV-specific T cells also had an elevation of CD4 + IL-17 + and CD8 + IFNγ, IL-17 + and TNFα + responses specific to SARS-CoV-2 peptides.
More evidence is needed to understand the mechanistic link between cytomegalovirus and SARS-CoV-2-specific T cell responses. To the best of our knowledge, this is the first study that focuses on anti-CMV humoral and cellular immune activity and its associations with SARS-CoV-2 T cell-specific immune profiles. Because we had no CMV-negative patients, it is not possible to attribute causality to the results obtained. Nonetheless, CMV-specific T cell responses in COVID-19 patients could be implicated in an immune polarization process during SARS-CoV-2 infection.
Limitations
The sample size was relatively small and restricted to a Brazilian cohort in this study, and it is not possible to confirm that high T responses to cytomegalovirus are the cause of SARS-CoV-2 immune dysregulation because all patients were CMV IgG-seropositive. Because all data stem from COVID-19 patients, it cannot be excluded that SARS-CoV-2 caused the different profiles of T cell responses to CMV. Also, additional data with respect to CMV- and SARS-CoV-2-specific Th2 cytokines in hiT CMV and loT CMV responders would give an important and comprehensive insight into the effect of CMV in T cell response to SARS-CoV-2. Due to the lack of differences between groups and an overall low frequency observed, we also did not mention in this study the presence of polyfunctional cells toward CMV and SARS-CoV-2 peptides.
Conclusions
COVID-19 patients with a high frequency of CMV-specific CD4 + and CD8 + cells exhibit a profile skewed toward CD4 + Th17 responses on stimulation with SARS-CoV-2 peptides, as well as broader specific CD8 + T cell activity reflected by IFNγ, IL-17, and TNFα production. Thus, CMV-amplified IL-17-dominated responses to SARS-CoV-2 could be further investigated as a mechanistic player for long-lasting damage as a result of maladjusted immunity from COVID-19 acute phase.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Wu S-E, Miller WE (2015) The human cytomegalovirus lytic cycle is induced by 1,25- dihydroxy vitamin D3 in peripheral blood monocytes and in the THP-1 monocytic cell line. Virology 483:83–95
Li X, Huang Y, Xu Z, Zhang R, Liu X, Li Y et al (2018) Cytomegalovirus infection and outcome in immunocompetent patients in the intensive care unit: A systematic review and meta-analysis. BMC Infect Dis 18(1):1–10
Lobato-Silva DF (2016) Citomegalovírus: epidemiologia baseada em dados de soroprevalência. Rev Pan-Amazônica Saúde. 7:213–9
Moura JU, Moraes GB, Capiotti MP, Silva RM, Leal DBR (2007) Prevalência Sorológica de Anti-CMV Em Gestantes da Região Oeste de Santa Maria, RS. Disc Scientia Ciências da Saúde, Santa Maria, Brazil. 8(1):33–9
Souza MA, Passos AM, Treitinger A, Spada C (2010) Seroprevalence of cytomegalovirus antibodies in blood donors in southern, Brazil | Soroprevalência de anticorpos contra citomegalovírus em doadores de sangue do Sul do Brasil. Rev Soc Bras Med Trop 43(4):359–361
Roberts ET, Haan MN, Dowd JB, Aiello AE (2010) Cytomegalovirus antibody levels, inflammation, and mortality among elderly latinos over 9 years of follow-up. Am J Epidemiol 172(4):363–371
Wang GC, Kao WHL, Murakami P et al (2010) Cytomegalovirus infection and the risk of mortality and frailty in older women: A prospective observational cohort study. Am J Epidemiol 171(10):1144–1152
Vescovini R, Biasini C, Telera AR et al (2010) Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol 184(6):3242–3249
Pera A, Campos C, Corona A et al (2014) CMV latent infection improves CD8+ T response to SEB due to expansion of polyfunctional CD57+ cells in young individuals. PLoS ONE 9(2):1–7
Pera A, Vasudev A, Tan C, Kared H, Solana R, Larbi A (2017) CMV induces expansion of highly polyfunctional CD4 + T cell subset coexpressing CD57 and CD154. J Leukoc Biol 101(2):555–566
Precopio ML, Betts MR, Parrino J et al (2007) Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med 204(6):1405–1416
Rocha CS, Hirao LA, Weber MG et al (2018) Subclinical cytomegalovirus infection is associated with altered host immunity, gut microbiota, and vaccine responses. J Virol 92(13):1–18
Salimi S, Hamlyn JM (2020) COVID-19 and Crosstalk With the Hallmarks of Aging. J Gerontol Ser A. 20:1–8
World Health Organization (2021) WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/
Fazolo T, Lima K, Fontoura JC et al (2021) Pediatric COVID-19 patients in South Brazil show abundant viral mRNA and strong specific anti-viral responses. Nat Commun 12(1):1–13
Stadlbauer D, Amanat F, Chromikova V, Krammer F (2020) SARS-CoV-2 seroconversion in humans a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol 57:1–15
Thakur B, Dubey P, Benitez J et al (2021) A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep. 11(1):1–13. https://doi.org/10.1038/s41598-021-88130-w
Honardoost M, Janani L, Aghili R, Emami Z, Khamseh ME (2021) The association between presence of comorbidities and COVID-19 severity: a systematic review and meta-analysis. Cerebrovasc Dis 50(2):132–140
Parry HM, Zuo J, Frumento G et al (2016) Cytomegalovirus viral load within blood increases markedly in healthy people over the age of 70 years. Immun Ageing. https://doi.org/10.1186/s12979-015-0056-6
D’Ardes D, Boccatonda A, Schiavone C et al (2020) A Case of Coinfection with SARS-COV-2 and cytomegalovirus in the Era of COVID-19. EJCRIM 7:1
Le Balc’h P, Pinceaux K, Pronier C, Seguin P, Tadié JM, Reizine F (2020) Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care 24(1):1–3
Jiwa N, Lorick K, Mutneja R (2020) Cytomegalovirus colitis post Il-6 inhibitor use in Covid-19 infection. Chest 158(4):A772. https://doi.org/10.1016/j.chest.2020.08.719
Leemans S, Maillart E, Van Noten H et al (2020) Cytomegalovirus hemorrhagic colitis complicating COVID-19 in an immunocompetent critically ill patient: A case report. Clin Case Reports. 9:1–5
Marchi G, Vianello A, Crisafulli E et al (2020) Cytomegalovirus-induced gastrointestinal bleeding and pancreatitis complicating severe COVID-19 pneumonia: a paradigmatic case. Mediterr J Hematol Infect Dis 12(1):12–15
Pitoyo CW, Wijaya IPEK, Wulani V, Wiraputri AK, Romulo MA (2020) Misleading diagnosis of radiological imaging of COVID-19 pneumonia during pandemic era: risk on the existence of CMV infection. Acta Med Indones 52(4):375–382
Moniz P, Brito S, Póvoa P (2021) SARS-CoV-2 and cytomegalovirus co-infections—a case series of critically ill patients. J Clin Med 10(13):2–6
Nenna R, Zhai J, Packard SE et al (2020) High cytomegalovirus serology and subsequent COPD-related mortality: a longitudinal study. ERJ Open Res. 6(2):00062–02020. https://doi.org/10.1183/23120541.00062-2020
Tan DAB, Amran FS, Teo TH, Price P, Moodley YP (2016) Levels of CMV-reactive antibodies correlate with the induction of CD28null T cells and systemic inflammation in chronic obstructive pulmonary disease (COPD). Cell Mol Immunol 13(4):551–3. https://doi.org/10.1038/cmi.2015.4
Li Z, Tang Y, Tang N et al (2017) High anti-human cytomegalovirus antibody levels are associated with the progression of essential hypertension and target organ damage in Han Chinese population. PLoS ONE 12(8):1–18
Wang H, Peng G, Bai J et al (2017) Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): A meta-analysis of prospective studies up to 2016. J Am Heart Assoc 6(7):1–10
Espinosa OA, Zanetti AS, Antunes EF, Longhi FG, Matos TA, Battaglini PF (2020) Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo 62(43):1–13
Veldhoen M (2017) Interleukin 17 is a chief orchestrator of immunity. Nat Immunol 18(6):612–621
Annunziato F, Romagnani C, Romagnani S (2015) The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 135(3):626–35. https://doi.org/10.1016/j.jaci.2014.11.001
Sadler AJ, Williams BRG (2008) Interferon-inducible antiviral effectors. Nat Rev Immunol 8(7):559–568
Lucas C et al (2020) Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584:463–469
Hussman JP (2021) Severe clinical worsening in COVID-19 and potential mechanisms of immune-enhanced disease. Front Med 8(June):1–13
Jahaj E, Vassiliou AG, Keskinidou C et al (2021) Evaluating the role of the interleukin-23/17 axis in critically ill COVID-19 patients. J Pers Med. 11(9):891
Hasan MZ, Islam S, Matsumoto K, Kawai T (2021) SARS-CoV-2 infection initiates interleukin-17-enriched transcriptional response in different cells from multiple organs. Sci Rep 11(1):1–11. https://doi.org/10.1038/s41598-021-96110-3
Hou W, Kang HS, Kim BS (2009) Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med 206(2):313–328
Knosp CA, Johnston JA (2011) Regulation of CD4+ T-cell polarization by suppressor of cytokine signaling proteins. Immunology 135:101–111
Vabret N, Britton GJ, Gruber C et al (2020) Immunology of COVID-19: current state of the science. Immunity 52(6):910–941
Naendrup JH, Borrega JG, Eichenauer DA, Shimabukuro-Vornhagen A, Kochanek M, Böll B (2021) Reactivation of EBV and CMV in severe COVID-19—epiphenomena or trigger of hyperinflammation in need of treatment? a large case series of critically ill patients. J Intensive Care Med. 37:1–7
Shrock E, Fujimura E, Kula T et al (2020) Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 370(6520):1–23
Jo N, Zhang R, Ueno H et al (2021) Aging and CMV infection affect pre-existing SARS-CoV-2-reactive CD8+ T cells in unexposed individuals. Front Aging 2:1–16
Furman D, Jojic V, Sharma S et al (2015) Cytomegalovirus infection improves immune responses to influenza. Sci Transl Med 7(281):1–22
Barton ES, White DW, Cathelyn JS et al (2007) Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447(7142):326–329
Nilsson C, Linde A, Montgomery SM et al (2005) Does early EBV infection protect against IgE sensitization? J Allergy Clin Immunol 116(2):438–444
Limaye AP, Boeckh M (2010) CMV in critically ill patients: Pathogen or bystander? Rev Med Virol 20(6):372–379
Weber S, Kehl V, Erber J, Busch DH (2021) CMV seropositivity is a potential novel risk factor for severe COVID-19 in non-geriatric patients. medRxiv 323:1–19
Mason GM, Poole E, Sissons JG, Wills MR, Sinclair JH (2012) Human cytomegalovirus latency alters the cellular secretome, inducing cluster of differentiation (CD)4+ T-cell migration and suppression of effector function. PNAS 109(36):14538–14543
Acknowledgements
We wish to thank Dr. Alessandra Peres, Dr. Gilson Dorneles, and all the colleagues from the immunotherapy laboratory for the technical support. We also thank all the patients that agreed to participate in this study.
Funding
This study was supported by PROADI, Moinhos de Vento Hospital, and the Ministry of Health. Fellowships for Fernanda Tereza Bovi Frozza, Priscila Oliveira de Souza, and Tiago Fazolo are from CAPES; fellowships for Karina Lima, Julia C. Fontoura, and Cristina Bonorino are from CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Edited by Matthias J. Reddehase.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frozza, F.T.B., Fazolo, T., de Souza, P.O. et al. A high CMV-specific T cell response associates with SARS-CoV-2-specific IL-17 T cell production. Med Microbiol Immunol 212, 75–91 (2023). https://doi.org/10.1007/s00430-022-00758-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-022-00758-1