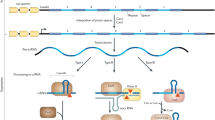
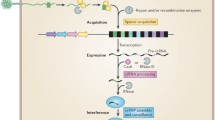
Abstract
Microbial cells are constantly threatened by invasion of foreign genetic elements. There are many mobile genetic elements (MGEs), including viruses, plasmids, transposons, etc., that invade them. Such selective pressure provoked the production of various defense systems in bacteria and other microorganisms, such as restriction–modification, abortive infection, exclusion of phage superinfection, toxin–antitoxin modules, argonaute proteins, and adaptive immune response. In this paper, we will consider the adaptive immunity systems of bacteria and archaea (CRISPR-Cas), their complex organization and functioning, the data on their origin associated with a new class of transposons, self-replicating casposons and other MGEs, and their role in the regulation of virulence genes and other important bacterial functions, as well as data on production in MGE systems that oppose adaptive immunity.
Similar content being viewed by others
REFERENCES
Barrangou, R. and Marraffini, L.A., CRISPR-Cas systems: Procaryotes upgrade to adaptive immunity, Mol. Cell, 2014, vol. 54, pp. 234–244. https://doi.org/10.1016/j.molcel.2014.03.011
Marraffini, L.A., CRISPR-Cas immunity in prokaryotes, Nature, 2015, vol. 526, pp. 55–61. https://doi.org/10.1038/nature15386
Mohanraju, P., Makarova, K.S., Zetsche, B., Zhang, F., Koonin, E.V., and van der Oost, J., Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems, Science, 2016, vol. 353, p. aad5147. https://doi.org/10.1126/science.aad5147
Amitai, G. and Sorek, R., CRISPR-Cas adaptation: insights into the mechanism of action, Nat. Rev. Microbiol., 2016, vol. 14, pp. 67–76. https://doi.org/10.1038/nrmicro.2015.14
Sternberg, S.H., Richter, H., Charpentier, E., and Qimron, U., Adaptation in CRISPR-Cas systems, Mol. Cell, 2016, vol. 61, pp. 797–808. https://doi.org/10.1016/j.molcel.2016.01.030
Makarova, K.S., Haft, D.H., Barrangou, R., Brouns, S.J., Charpentier, E., Horvath, P., et al., Evolution and classification of the CRISPR-Cas systems, Nat. Rev. Microbiol., 2011, vol. 9, no. 6, pp. 467–477. https://doi.org/10.1038/nrmicro2577
Makarova, K.S., Wolf, Y.I., and Koonin, E.V., Classification and nomenclature of CRISPR-Cas systems: where from here?, CRISPR J., 2018, vol. 1, no. 5, pp. 325–326. https://doi.org/10.1089/crispr.2018.0033
Makarova, K.S., Wolf, Y.I., Iranzo, S.A., Shmakov, S.A., Alkhnbashi, O.S., Brouns, S.J.J., et al., Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants, Nat. Rev. Microbiol., 2020, vol. 18, no. 2, pp. 67–83. https://doi.org/10.1038/s41579-019-0299-x
Amitai, G. and Sorek, R., CRISPR-Cas adaptation: insight into the mechanism of action, Nat. Rev. Microbiol., 2016, vol. 14, pp. 67–76. https://doi.org/10.1038/nrmicro.2015.14
Jackson, S.A., McKenzie, R.E., Fagerlund, R.D., Kieper, S.N., Fineran, P.C., and Brouns, S.I., CRISPR-Cas: adapting to change, Science, 2017, vol. 356, p. eaal5056. https://doi.org/10.1126/science.aal5056
Killelea, T. and Bolt, E.L., CRISPR-Cas adaptive immunity and the three Rs, Biosci. Rep., 2017, vol. 37, no. 4, p. BSR20160297. https://doi.org/10.1042/BSR20160297
Liu, I., Liu, Zh., Ye, Q., Pan, S., Wang, X., Li, Y., et al., Coupling transcriptional activation of CRISPR-Cas system and DNA repair genes by Csa3a in Sulfolobus islandicus, Nucleic Acids Res., 2017, vol. 45, pp. 8978–8992. https://doi.org/10.1093/nar/gkx612
Koonin, E.V. and Makarova, K.S., Origin and evolution of CRISPR-Cas systems, Philos. Trans. R. Soc. London, Ser. B, 2019, vol. 374, no. 1772, p. 20180087. https://doi.org/10.1098/rstb.20180087
Charpentier, E., Richter, H., van der Oost, J., and White, M.F., Biogenesis pathways of RNA guides in archaeal and bacterial CRISPR-Cas adaptive immunity, FEMS Microbiol. Rev., 2015, vol. 39, pp. 428–441. https://doi.org/10.1093/femsre/fuv023
Nishimassu, H. and Nureki, O., Structure and mechanisms of CRISPR RNA-guided effector nucleases, Curr. Opin. Struct. Biol., 2017, vol. 43, pp. 68–78. https://doi.org/10.1016/j.sbi.2016.11.013
Plagens, A., Richter, H., Charpenter, E., and Randau, L., DNA and RNA interference mechanisms by CRISPR-Cas surveillance complexes, FEMS Microbiol. Rev., 2015, vol. 39, pp. 442–463. https://doi.org/10.1093/femsre/fuv019
Makarova, K.S., Wolf, Y.I., Alkhnbashi, O.S., Costa, F., Shah, S.A., Saunders, S.J., et al., An updated evolutionary classification of CRISPR-Cas systems, Nat. Rev. Microbiol., 2015, vol. 13, pp. 722–736. https://doi.org/10.1038/nrmicro3569
Koonin, E.V. and Makarova, K.S., Mobile genetics elements and evolution of CRISPR-Cas systems: all the way there and back, Genome Biol. Evol., 2017, vol. 9, pp. 2812–2825. https://doi.org/10.1093/gbe/evx192
Shmakov, S., Smargon, A., Scott, D., Cox, D., Pyzocha, N., Yan, W., et al., Diversity and evolution of class 2 CRISPR-Cas systems, Nat. Rev. Microbiol., 2017, vol. 15, no. 3, pp. 168–182. https://doi.org/10.1038/nrmicro.2016.184
Shmakov, S., Abudayyeh, O.O., Makarova, K.S., Wolf, Y.I., Gootenberg, J.S., Semenova, E., et al., Discovery and functional characterization of diverse class 2 CRISPR-Cas systems, Mol. Cell, 2015, vol. 60, pp. 385–397. https://doi.org/10.1016/j.molcel.2015.10.008
Krupovic, M., Makarova, K.S., Forterre, P., Prangishvili, D., and Koonin, E.V., Casposons: a new superfamily of self-synthesizing DNA transposons at the origin of prokaryotic CRISPR-Cas immunity, BMC Biol., 2014, vol. 19, pp. 12–36. https://doi.org/10.1186/1741-7007-12-36
Hickman, A.B. and Dyda, F., CRISPR-Cas immunity and mobile DNA: a new superfamily of DNA transposons encoding a Cas1 endonuclease, Mobile DNA, 2014, vol. 5, pp. 23–27. https://www.mobilednajournal.com/content/5/1/23.
Krupovic, M. and Koonin, E.V., Self-synthesizing transposons: unexpected key players in the evolution of viruses and defense systems, Curr. Opin. Microbiol., 2016, vol. 31, pp. 25–33. https://doi.org/10.1016/j.mib.2016.01.006
Koonin, E.V. and Krupovic, M., Evolution of adaptive immunity from transposable elements combined with innate immune systems, Nat. Rev. Genet., 2015, vol. 16, pp. 184–192. https://doi.org/10.1038/nrg3859
Krupovic, M., Beguin, P., and Koonin, E.V., Casposons: mobile genetic elements that gave rise to the CRISPR-Cas adaptation machinery, Curr. Opin. Microbiol., 2017, vol. 38, pp. 36–43. https://doi.org/10.1016/j.mib.2017.04.004
Hickman, A.B. and Dyda, F., The casposon-encoded Cas1 protein from Aciduliprofundum boonei is a DNA integrase that generates target site duplications, Nucleic Acids Res., 2015, vol. 43, pp. 10576–10587. https://doi.org/10.1093/nar/gkv1180
Beguin, P., Charpin, N., Koonin, E.V., Forterre, P., and Krupovic, M., Casposon integration shows strong target site preference and recapitulates protospacer integration by CRISPR-Cas systems, Nucleic Acids Res., 2016, vol. 44, pp. 10367–10376. https://doi.org/10.1093/nar/gkv821
Krupovic, M., Shmakov, S., Makarova, K.S., Forterre, P., and Koonin, E.V., Recent mobility of casposons, self-synthesizing transposons at the origin of the CRISPR-Cas immunity, Genome Biol. Evol., 2016, vol. 8, pp. 375–386. https://doi.org/10.1093/gbe/evw006
Makarova, K.S., Krupovic, M., and Koonin, E.V., Evolution of replicative DNA polymerases in archaea and their contribution to the eucaryotic replication machinery, Front. Microbiol., 2014, vol. 5, pp. 354. https://doi.org/10.3389/fmicb.2014.00354
Krupovic, M. and Koonin, E.V., Polintons: a hotbed of eukaryotic virus, transposon and plasmid evolution, Nat. Rev. Microbiol., 2015, vol. 13, pp. 105–115. https://doi.org/10.1038/nrmicro3389
Yutin, N., Backstrom, D., Ettema, T.J.G., Krupovic, M., and Koonin, V., Vast diversity of prokaryotic virus genomes encoding double jelly-roll major capsid proteins uncovered genomic and metagenomic sequence analysis, Virol. J., 2018, vol. 15, p. 67. https://doi.org/10.1186/s12985-018-0974-y
Krupovic, M., Makarova, K.S., Forterre, P., and Koonin, E.V., Recent mobility of casposons, self-synthesizing transposons at the origin of the CRISPR-Cas immunity, Genome Biol. Evol., 2018, vol. 8, no. 2, pp. 375–386. https://doi.org/10.1093/gbe/evw006
Koonin, E.V. and Krupovic, M., A movable defense, Scientist, 2015, vol. 29, no. 1, pp. 46–53.
Makarova, K.S., Grishin, N.V., Shabalina, S.A., Wolf, Y.I., and Koonin, E.V., A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action, Biol. Direct, 2006, vol. 1, p. 7. https://doi.org/10.1186/1745-6150-1-7
Wiedenheft, B., Zhou, K., Jinek, M., Coyle, S.M., Ma, W., and Doudna, J.A. Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense, Structure, 2009, vol. 17, pp. 904–912. https://doi.org/10.1016/j.str.2009.03.019
Nunez, J.K., Kranzusch, P.J., Noeske, J., Wright, A.V., Davies, C.W., and Doudna, J.A., Cas1–Cas2 complex formation mediates spacer acquisition during CRISPR-Cas adaptive immunity, Nat. Struct. Mol. Biol., 2014, vol. 21, pp. 528–534. https://doi.org/10.1038/nsmb.2820
Nunez, J.K., Lee, A.S., Engelman, A., and Doudna, J.A., Integrase-mediated spacer acquisition during CRISPR-Cas adaptive immunity, Nature, 2015, vol. 519, pp. 193–198. https://doi.org/10.1038/nature14237
Arslan, Z., Hermanns, V., Wurm, R., Wagner, R., and Pul, U., Detection and characterization of spacer integration intermediates in type I-E CRISPR-Cas system, Nucleic Acids Res., 2014, vol. 42, pp. 7884–7893. https://doi.org/10.1093/nar/gkv510
Nunez, J.K., Kranzusch, P.J., Noeske, J., Wright, A.V., Davies, C.W., and Doudna, J.A., Cas1–Cas2 complex formation mediates spacer acquisition during CRISPR-Cas adaptive immunity, Nat. Struct. Mol. Biol., 2014, vol. 21, pp. 528–534. https://doi.org/10.1038/nsmb.2820
Faure, G., Makarova, K.S., and Koonin, E.V., CRISPR-Cas: complex functional networks and multiple roles beyond adaptive immunity, J. Mol. Biol., 2019, vol. 431, no. 1, pp. 3–20. https://doi.org/10.1016/j.jmb.2018.08.030
Sampson, T.R. and Weiss, D.S., CRISPR-Cas systems: new players in gene regulation and bacterial physiology, Front. Cell. Infect. Microbiol., 2014, vol. 4, p. 37. https://doi.org/10.3389/fcimb.2014.00037
Sampson, T.R., Saroj, S.D., Llewellyn, A.C., Tzeng, Y.L., and Weiss, D.S., A CRISPR-Cas system mediates bacterial innate evasion and virulence, Nature, 2013, vol. 497, pp. 254–257. https://doi.org/10.1038/nature12048
Louwen, R., Staals, R.H., Endtz, H.P., van Baarlen, P., and van der Oost, J., The role of CRISPR-Cas system in virulence of pathogenic bacteria, Microbiol. Mol. Biol. Rev., 2014, vol. 78, pp. 74–88. https://doi.org/10.1128/MMBR.00039-13
Watson, B.N.J., Staals, R.H.J., and Fineran, P.C., CRISPR-Cas-mediated phage resistance enhances horizontal gene transfer by transduction, mBio, 2018, vol. 9, no. 1, p. e02406-17. https://doi.org/10.1128/mBio.02406-17
Goldberg, G.W., Jiang, W., Bikard, D., and Marraffini, L.A., Conditional tolerance of temperate phages via transcription-dependent CRISPR-Cas targeting, Nature, 2014, vol. 514, pp. 633–637. https://doi.org/10.1038/nature13637
Makarova, K.S., Aravind, L., Grishin, N.V., Rogozin, I.B., and Koonin, E.V., A DNA repair system specific for thermophilic Archaea and bacteria predicted by genomic context analysis, Nucleic Acids Res., 2002, vol. 30, pp. 482–496. https://doi.org/10.1093/nar/30.2.482
Killelea, T. and Bolt, E.I., CRISPR-Cas adaptive immunity and the three Rs, Biosci. Rep., 2017, vol. 37, p. BSR20160297. https://doi.org/10.1042/BSR20160297
Liu, T., Liu, Z., Ye, Q., Pan, S., Wang, X., Li, Y., et al., Coupling transcriptional activation of CRISPR-Cas system and DNA repair genes by Cas3a in Sulfolobus islandicus, Nucleic Acids Res., 2017, vol. 45, pp. 8978–8992. https://doi.org/10.1093/nar/gks612
Ratner, H.K., Sampson, T.R., and Weiss, D.S., I can see CRISPR now, even when phage are gone: a view on alternative CRISPR-Cas functions from prokaryotic envelope, Curr. Opin. Infect. Dis., 2015, vol. 28, no. 3, pp. 267–274. https://doi.org/10.1097/QCO.0000000000000154
Sampson, T.R., Napier, B.A., Schroeder, M.R., Louwen, R., Zhao, J., Chin, C.Y., et al., A CRISPR-Cas system enhances envelope integrity mediating resistance and inflammasome evasion, Proc. Natl. Acad. Sci. U. S. A., 2014, vol. 111, pp. 11163–11168. https://doi.org/10.1073/pnas.1323025111
Louwen, R., Horst-Kreft, D., De Boer, A.G., Van Der Graaf, L., De Knegt, G., Hamersma, M., et al., A novel link between Compylobacter jejuni bacteriophage defense, virulence and Guillain–Barre syndrome, Eur. J. Clin. Microbiol. Infect. Dis., 2013, vol. 32, pp. 207–226. https://doi.org/10.1007/s10096-012-1733-4
Perez-Rodriguez, R., Haitjema, C., Huang, Q., Nam, K., Bernardis, S., Ke, A., et al., Envelope stress is a trigger of CRISPR RNA-mediated DNA silencing in Escherichia coli, Mol. Microbiol., 2011, vol. 79, pp. 584–599. https://doi.org/10.1111/j.1365-2958.2010.07265.x
Young, J.C., Dill, B.D., Pan, C., Hettich, R.L., Banfield, J.F., Shah, M., et al., Phage-induced expression of CRISPR-associated proteins is revealed by shotgun proteomics in Streptococcus thermophilus, PLoS One, 2012, vol. 7, p. e38077. https://doi.org/10.1371/journal.pone.0038077
Gunderson, F.F. and Cianciotto, N.P., The CRISPR-associated gene cas2 of Legionella pneumophila is required for intracellular infection of amoebae, mBio, 2013, vol. 4, p. e00074-13. https://doi.org/10.1128/mBio.00074-13
Bourgogne, A., Garsin, D.A., Qin, X., Singh, K.V., Sillanpaa, J., Yerrapragada, S., et al., Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OGIRF, Genome Biol., 2008, vol. 9, p. R110. https://doi.org/10.1186/gb-2008-9-7-r110
Zegans, M.E., Wagner, J.C., Cady, K.C., Murphy, D.M., Hammond, J.H., and O’Toole, G.A., Interaction between bacteriophage DM53 and host CRISPR region inhibits group behaviors of Pseudomonas aeruginosa, J. Bacteriol., 2009, vol. 191, pp. 210–219. https://doi.org/10.1128/JB.00797-08
Palmer, K.L. and Whiteley, M., DMS3-42: the secret to CRISPR-dependent biofilm inhibition in Pseudomonas aeruginosa, J. Bacteriol., 2011, vol. 193, pp. 3431–3432. https://doi.org/10.1128/JB.05066-11
Makarova, K.S., Anantharaman, S., Aravind, L., and Koonin, E.V., Live virus—free or die: coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes, Biol. Direct, 2012, vol. 7, pp. 40–50. https://www.biologyodirect.com/content/7/1/40.
Makarova, K.S., Wolf, Y.I., and Koonin, E.V., Comparative genomics of defense systems in archaea and bacteria, Nucleic Acids Res., 2013, vol. 41, pp. 4360–4377. https://doi.org/10.1093/nar/gkt157
Koonin, E.V. and Zhang, F., Coupling immunity and programmed cell suicide in prokaryotes: life or death choices, Bioassays, 2017, vol. 39, pp. 1–9. https://doi.org/10.1002/bies.201600186
Peters, J.E., Makarova, K.S., Shmakov, S., and Koonin, E.V., Recruitment of CRISPR-Cas systems by Tn7-like transposon, Proc. Natl. Acad. Sci. U. S. A., 2017, vol. 114, pp. 7358–7366. https://doi.org/10.1073/pnas.1709035114
Seed, K.D., Lazinski, D.W., Calderweed, S.B., and Camilli, A., A bacteriophage encodes its own CRISPR/ Cas adaptive response to evade host innate immunity, Nature, 2013, vol. 494, pp. 489–491. https://doi.org/10.1038/nature11927
Villion, M. and Moineau, S., Virology: phages hijack a host defense, Nature, 2013, vol. 494, pp. 433–434. https://doi.org/10.1038/494433a
Pinilla-Redonda, R., Shehreen, S., Marino, N.D., Fagerlund, R.D., Brown, Ch.M., Sorensen, S.J., et al., Discovery of multiple anti-CRISPRs highlights anti-defense gene clustering in mobile genetic elements, Nat. Commun., 2020, vol. 11, p. 5652. https://doi.org/10.1038/s41467-020-19415-3
Faure, G., Shmakov, S.A., Yan, W.X., Cheng, D.R., Scott, D.A., Peters, J.E., et al., CRISPR-Cas in mobile genetic elements: counter-defense and beyond, Nat. Rev. Microbiol., 2019, vol. 17, no. 8, pp. 513–525. https://doi.org/10.1038/s41579-019-0204-7
Borges, A.L., Davidson, A.R., and Bondy-Denomy, J., The discovery, mechanisms, and evolutionary impact of anti-CRISPRs, Annu. Rev. Virol., 2018, vol. 4, pp. 32–39. https://doi.org/10.1146/annurev-virology-101416-041616
Bondy-Denomy, J., Pawluk, A., Maxwell, K.L., and Davidson, A.R., Bacteriophage genes that inactivate the CRISPR-Cas bacterial immune system, Nature, 2013, vol. 493, pp. 429–432. https://doi.org/10.1038/nature11723
Watters, K.E., Fellmann, C., Bai, H.B., Ren, S.M., and Doudna, J.A., Systematic discovery of natural CRISPR-Cas12a inhibitors, Science, 2018, vol. 362, pp. 236–239. https://doi.org/10.1126/science.aau5138
Mahendra, C., Christie, K.A., Osuna, B.A., Redondo, R., Kleinstiver, B.P., Bondy-Denomy, J., et al., Broad spectrum anti-CRISPR proteins facilitate horizontal gene transfer, Nat. Microbiol., 2020, vol. 5, pp. 620–629. https://doi.org/10.1038/s41564-020-0726-9
Trasanidou, D., Geros, A.S., Mohanraji, P., Nieuwenweg, C., Nobrega, F.L., and Staals, R.H.J., Keeping CRISPR in check: diverse mechanisms of phage-encoded anti-CRISPRS, FEMS Microbiol. Lett., 2019, vol. 366, p. fnz098. https://doi.org/10.1093/femsle/fnz098
Birkholz, N., Fagerlund, R.D., Smith, I.M., Jackson, S.A., and Fineran, P.C., The autoregulator Aca2 mediates anti-CRISPR repression, Nucleic Acids Res., 2019, vol. 47, pp. 9658–9665. https://doi.org/10.1093/nar/gkz721
Pawluk, A., Staals, R.H.J., Taylor, C., Watson, B.N.J., Saha, S., Fineran, P.C., et al., Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species, Nat. Microbiol. J., 2016, vol. 1, p. 16085. https://doi.org/10.1038/nmicrobiol.2016.85
Stanley, S.Y., Borges, A.L., Chen, K.-H., Swaney, D.L., Kroyan, N.J., Bondy-Denomy, J., and Davidson, A.R., Anti-CRISPR-associated proteins are crucial repressors of anti-CRISPR transcription, Cell, 2019, vol. 178, pp. 1452–1464. https://doi.org/10.1016/j.cell.2019.07.046
Davidson, A.R., Lu, W.T., Stanley, S.Y., Wang, J., Mejdani, M., Trost, C.N., et al., Anti-CRISPR inhibitors of CRISPR-Cas systems, Annu. Rev. Biochem., 2020, vol. 89, pp. 309–332. https://doi.org/10.1146/annurev-biochem-011420-111224
Leon, L.M., Park, A.E., Borges, A.L., Zhang, J.Y., and Bondy-Denomy, J., Mobile element warfare via CRISPR and anti-CRISPR in Pseudomonas aeruginosa, Nucleic Acids Res., 2021, vol. 49, no. 4, pp. 2114–2125. https://doi.org/10.1093/nar/gkab006
Marino, N.D., Pinilla-Redondo, R., Csorgo, B., and Bondy-Denomy, J., Anti-CRISPR protein applications: natural brakes for CRISPR-Cas technologies, Nat. Methods, 2020, vol. 17, no. 5, pp. 471–479. https://doi.org/10.1038/s41592-020-0771-6
Funding
There is no funding for the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human beings performed by the author.
Conflict of Interest
The author declares that he has no conflicts of interest.
Additional information
Translated by D. Novikova
About this article
Cite this article
Ilyina, T.S. Adaptive Immunity Systems of Bacteria: Association with Self-Synthesizing Transposons, Polyfunctionality. Mol. Genet. Microbiol. Virol. 37, 117–126 (2022). https://doi.org/10.3103/S0891416822030065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416822030065