Abstract
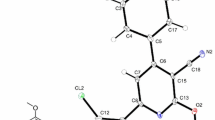
Strychnine is well known as a highly toxic alkaloid derived from the plant Strychnos nux-vomica. It has been applied for chiral separations as a resolving agent for co-crystallizations, as a standard for chromatographic separations, as a rodenticide, as a natural therapy and stimulant, and as a poisonous plot device in works of fiction. Dissolving strychnine into the common organic solvent dichloromethane results in the spontaneous crystallization of the title compound N-(chloromethyl)strychninium chloride. Crystals of the compound were isolated in the orthorhombic space group P212121 with a = 7.6819(2) Å, b = 7.9621(2) Å, c = 30.7170(9) Å, α = 90°, β = 90°, γ = 90°. The crystal structure has bilayers of N-(chloromethyl)strychninium cations with corresponding bilayers of chloride ions interacting to the cations through C–H hydrogen bonds.
Graphical Abstract






Similar content being viewed by others
Data Availability
CCDC-1,815,572 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge at http://www.ccdc.cam.ac.uk/ or from the Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44(0)1223-336033; email: deposit@ccdc.cam.ac.uk.
References
Buckingham J (2007) Bitter nemesis: the intimate history of strychnine. CRC Press, Boca Ratonhttps://doi.org/10.1201/9781420053166
McGarry RC, McGarry P (1999) Please pass the strychnine: the art of victorian pharmacy. Can Med Assoc J 161(12):1556–1558
Rosen DM (2008) Dope: a history of performance enhancement in sports from the nineteenth century to today. Praeger, Westport
Harris T, Sport (2008) Almost everything you ever wanted to know. Yellow Jersey, London
Mallon B, Heijmans J (2011) Historical dictionary of the olympic movement, historical dictionaries of sports, 4th edn. The Scarecrow Press, Plymouth
Akbar S, Khan SA, Masood A, Iqbal M (2010) Use of Strychnos Nux-Vomica (azraqi) seeds in unani system of medicine: role of detoxification. Afr J Tradit Complement Altern Med 7(4):286–290. https://doi.org/10.4314/ajtcam.v7i4.56689
State Pharmacopoeia Commission of the People’s Republic of China (2015) Pharmacopoeia of the people’s Republic of China. Chemical Industry Press, Beijing
Wang Y, Chen A (2013) Crystallization-based separation of enantiomers. In: Andrushko V, Andrushko N (eds) Stereoselective synthesis of drugs and natural products. Wiley, Hobokenhttps://doi.org/10.1002/9781118596784.ssd056
Jaen JC (2003) Brucine. In: Paquette LA (ed) Chiral reagents for asymmetric synthesis: handbook of reagents for organic synthesis. Wiley, New York
Smith G, Wermuth UD, Williams ML (2012) Resolution of the chiral (1R,2S) enantiomer of cis-cyclohexane-1,2-dicarboxylic acid in the brucinium salt 2,3-dimethoxy-10-oxostrychnidinium (1R,2S)-2-carboxycyclohexane-1-carboxylate dihydrate. J Chem Crystallogr 42(6):555–559. https://doi.org/10.1007/s10870-012-0278-9
Brands KMJ, Davies AJ (2006) Crystallization-induced diastereomer transformations. Chem Rev 106(7):2711–2733. https://doi.org/10.1021/cr0406864
Philippe G, Angenot L, Tits M, Frederich M (2004) About the toxicity of some Strychnos species and their alkaloids. Toxicon 44(4):405–416. https://doi.org/10.1016/j.toxicon.2004.05.006
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge structural database. Acta Crystallogr B 72:171–179. https://doi.org/10.1107/S2052520616003954
Ma FS, Wu XJ, Yu Y, Tong SQ, Liang Y, Fang JQ et al (2016) Preparative separation and purification of two highly polar alkaloids derived from Semen Strychni extracted with dichloromethane by high-speed countercurrent chromatography. J Sep Sci 39(19):3709–3715. https://doi.org/10.1002/jssc.201600352
Sheldrick GM (2015) SHELXT: integrated space-group and crystal-structure determination. Acta Crystallogr A 71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71:3–8. https://doi.org/10.1107/S2053229614024218
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Cottrell SJ, Olsson TSG, Taylor R, Cole JC, Liebeschuetz JW (2012) Validating and understanding ring conformations using small molecule crystallographic data. J Chem Inf Model 52(4):956–962. https://doi.org/10.1021/ci200439d
Bruno IJ, Cole JC, Kessler M, Luo J, Motherwell WDS, Purkis LH et al (2004) Retrieval of crystallographically-derived molecular geometry information. J Chem Inf Comp Sci 44(6):2133–2144. https://doi.org/10.1021/ci049780b
Aakeröy CB, Evans TA, Seddon KR, Pálinkó I (1999) The C–H···Cl hydrogen bond: does it exist? New J Chem 23(2):145–152. https://doi.org/10.1039/A809309A
Thallapally PK, Nangia A (2001) A Cambridge structural database analysis of the C-H···Cl interaction: C-H···Cl- and C-H···Cl-M often behave as hydrogen bonds but C-H···Cl-C is generally a van der Waals interaction. CrystEngComm. https://doi.org/10.1039/b102780h
Berkman CE, Fernandez EJ, Thompson CM, Pavkovic SF (1993) Structure of 19-methylstrychninium (S)-(S-[(R)-1,2-Di(ethoxycarbonyl)ethyl] O-methyl phosphorodithioate). Acta Crystallogr C 49:554–556. https://doi.org/10.1107/S0108270192007637
Gould RO, Walkinshaw MD (1984) Molecular recognition in model crystal complexes: the resolution of D-Amino and L-Amino-acids. J Am Chem Soc 106(25):7840–7842. https://doi.org/10.1021/ja00337a031
Mostad A (1985) Structural study of the strychnine molecule in crystals of the free base and of the nitric-acid complex. Acta Chem Scand B 39(9):705–716. https://doi.org/10.3891/acta.chem.scand.39b-0705
Ghosh R, Roychowdhury P, Chattopadhyay D, Iitaka Y (1989) Structure of strychnine hydrochloride sesquihydrate. Acta Crystallogr C 45:1794–1797. https://doi.org/10.1107/s0108270189003483
Bialonska A, Ciunik Z (2005) Crystal structure of strychninium chloride dihydrate: hidden helix in the water/anion tape. J Mol Struct 779(1–3):38–42. https://doi.org/10.1016/j.molstruc.2005.07.007
Berger S, Sicker D, Strychnine (2009) Classics in spectroscopy. Wiley-VCH Verlay GmbH & Co, Weinheim, pp 103–128
Klemperer ME, Warren FL (1955) Chem Ind 1955:1553
Caws AC, Foster GE (1956) A source of error in the assay of strychnine salts and preparations containing strychnine. J Pharm Pharmacol 8(10):790–798. https://doi.org/10.1111/j.2042-7158.1956.tb12210.x
Caws AC, Foster GE (1957) The purity of Chloroform Bp. J Pharm Pharmacol 9(12):824–833. https://doi.org/10.1111/j.2042-7158.1957.tb12343.x
Coomber DI, Rose BA (1959) The reaction of bases with chloroform. J Pharm Pharmacol 11(11):703–704. https://doi.org/10.1111/j.2042-7158.1959.tb12616.x
Phillipson JD, Bisset NG (1972) Artifacts produced by Chloroform and Methylene Dichloride during extraction of Amines and Alkaloids.1. Quaternization and oxidation of strychnine and brucine during plant extraction. Phytochemistry 11(8):2547–2553. https://doi.org/10.1016/S0031-9422(00)88534-9
Shi YS, Liu YB, Ma SG, Li L, Qu J, Li Y et al (2014) Four new minor alkaloids from the seeds of Strychnos nux-vomica. Tetrahedron Lett 55(48):6538–6542. https://doi.org/10.1016/j.tetlet.2014.09.127
Lazareno S, Gharagozloo P, Kuonen D, Popham A, Birdsall NJM (1998) Subtype-selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding studies. Mol Pharmacol 53(3):573–589. https://doi.org/10.1124/mol.53.3.573
Gharagozloo P, Lazareno S, Popham A, Birdsall NJM (1999) Allosteric interactions of quaternary strychnine and brucine derivatives with muscarinic acetylcholine receptors. J Med Chem 42(3):438–445. https://doi.org/10.1021/jm970799y
Birdsall NJM, Farries T, Gharagozloo P, Kobayashi S, Lazareno S, Sugimoto M (1999) Subtype-selective positive cooperative interactions between brucine analogs and acetylcholine at muscarinic receptors: functional studies. Mol Pharmacol 55(4):778–786
Griffin MT, Hsu JCH, Shehnaz D, Ehlert FJ (2003) Comparison of the pharmacological antagonism of M-2 and M-3 muscarinic receptors expressed in isolation and in combination. Biochem Pharmacol 65(8):1227–1241. https://doi.org/10.1016/S0006-2952(03)00068-6
Jensen AA, Gharagozloo P, Birdsall NJM, Zlotos DP (2006) Pharmacological characterisation of strychnine and brucine analogues at glycine and alpha 7 nicotinic acetylcholine receptors. Eur J Pharmacol 539(1–2):27–33. https://doi.org/10.1016/j.ejphar.2006.04.010
Acknowledgements
The authors acknowledge the muwinina people, the traditional owners of the land on which this research was conducted. The authors acknowledge the Central Science Laboratory at the University of Tasmania for access to their services. Additional unpublished datasets of the title compound were collected on the MX1 beamline at the Australian Synchrotron, part of ANSTO.
Funding
This research was supported under the Australian Research Council’s Discovery Early Career Research Award funding scheme (Project Number DE150100263).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taylor, C.M., Kilah, N.L. The Structure of N-(Chloromethyl)Strychninium Chloride, a Spontaneously Crystallized Product Relevant to Analytical Chemistry, Bioprospecting, and Chiral Separations. J Chem Crystallogr 53, 379–385 (2023). https://doi.org/10.1007/s10870-022-00977-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-022-00977-7