Abstract
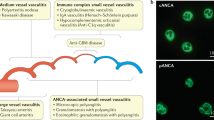
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a systemic autoimmune disease that is prone to respiratory and renal failures. Its major target antigens are serine protease 3 (PR3) and myeloperoxidase (MPO), but the determinants of PR3 and MPO subtypes are still unclear. Uncoupling protein-1 (UCP-1) and adropin (Adr) regulate mutually and play an important role in endothelial cell injury. In this study, adropin and UCP-1 knockout (AdrKO and UCP-1-KO) models were established on the basis of C57BL/6 J mice. The results showed that UCP-1-KO and AdrKO mice similar to AAV: significant inflammatory cell infiltration, vascular wall damage, and erythrocyte extravasation. The pathological basis of AdrKO was that endothelial cells adhered and activated neutrophils to release MPO, and the core gene was peroxisome proliferator–activated receptor gamma (PPARG). However, UCP-1-KO induced PR3 release, and the accumulation and expression of tissue factor on the vascular wall, and the core gene was peroxisome proliferator–activated receptor delta (PPARD). The present study verified that the subtypes of AAV may be genetically different diseases and it also provide novel experimental evidence for clinical differentiation of the two subtypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data used to support the findings of this study are currently under embargo while the research findings are commercialized. Requests for data, [12 months] after publication of this article, will be considered by the corresponding author.
References
Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32.
Nakaya I, Yahata M, Takahashi S, Sasajima T, Sakuma T, Shibagaki Y, et al. Long-term outcome and efficacy of cyclophosphamide therapy in Japanese patients with ANCA-associated microscopic polyangiitis: a retrospective study. Intern Med. 2013;52:2503–9.
Zhang S, Shu X, Tian X, Chen F, Liu X, Wang G. Enhanced formation and impaired degradation of neutrophil extracellular traps in dermatomyositis and polymyositis: a potential contributor to interstitial lung disease complications. Clin Exp Immunol. 2014;177:134–41.
Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10:463–73.
Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–23.
Cid MC. The search for genetic links in ANCA-associated vasculitis and its variants. N Engl J Med. 2012;367:271–3.
Knight A, Sandin S, Askling J. Risks and relative risks of Wegener’s granulomatosis among close relatives of patients with the disease. Arthritis Rheum. 2008;58:302–7.
Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–92.
Monach PA, Merkel PA. Genetics of vasculitis. Curr Opin Rheumatol. 2010;22:157–63.
Gao F, Fang J, Chen F, Wang CD, Chen S, Zhang S, et al. Enho mutations causing low adropin: a possible pathomechanism of MPO-ANCA associated lung injury. EBioMedicine. 2016;9:324–35.
Chen S, Zeng K, Liu QC, Guo Z, Zhang S, Chen XR, et al. Adropin deficiency worsens HFD-induced metabolic defects. Cell Death Dis. 2017;8:e3008.
Altintas O, Kumas M, Altintas MO. Neuroprotective effect of ischemic preconditioning via modulating the expression of adropin and oxidative markers against transient cerebral ischemia in diabetic rats. Peptides. 2016;79:31–8.
Holguin F, Ramadan B, Gal AA, Roman J. Prognostic factors for hospital mortality and ICU admission in patients with ANCA-related pulmonary vasculitis. Am J Med Sci. 2008;336:321–6.
Aydin S, Kuloglu T, Aydin S, Eren MN, Yilmaz M, Kalayci M, et al. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol Cell Biochem. 2013;380:73–81.
Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8:468–81.
Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–6.
Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–17.
Hong J, Liu R, Chen L, Wu BW, Yu J, Gao W, et al. Conditional knockout of tissue factor pathway inhibitor 2 in vascular endothelial cells accelerates atherosclerotic plaque development in mice. Thromb Res. 2016;137:148–56.
Manoharan I, Suryawanshi A, Hong Y, Ranganathan P, Shanmugam A, Ahmad S, et al. Homeostatic PPARα signaling limits inflammatory responses to commensal microbiota in the intestine. J Immunol. 2016;196:4739–49.
Besse-Patin A, Léveillé M, Oropeza D, Nguyen BN, Prat A, Estall JL. Estrogen signals through peroxisome proliferator-activated receptor-γ coactivator 1α to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology. 2017;152:243–56.
Luz-Crawford P, Ipseiz N, Espinosa-Carrasco G, Caicedo A, Tejedor G, Toupet K, et al. PPARβ/δ directs the therapeutic potential of mesenchymal stem cells in arthritis. Ann Rheum Dis. 2016;75:2166–74.
Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–59.e5.
Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–95.
Chen M, Kallenberg CG. ANCA-associated vasculitides—advances in pathogenesis and treatment. Nat Rev Rheumatol. 2010;6:653–64.
Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–56.
Kumar AP, Piedrafita FJ, Reynolds WF. Peroxisome proliferator-activated receptor gamma ligands regulate myeloperoxidase expression in macrophages by an estrogen-dependent mechanism involving the -463GA promoter polymorphism. J Biol Chem. 2004;279:8300–15.
Shirai T, Hilhorst M, Harrison DG, Goronzy JJ, Weyand CM. Macrophages in vascular inflammation—from atherosclerosis to vasculitis. Autoimmunity. 2015;48:139–51.
Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, et al. Adropin is a novel regulator of endothelial function. Circulation. 2010;122:S185–92.
Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9.
Williams JW, Elvington A, Ivanov S, Kessler S, Luehmann H, Baba O, et al. Thermoneutrality but Not UCP1 deficiency suppresses monocyte mobilization into blood. Circ Res. 2017;121:662–76.
Ramage LE, Akyol M, Fletcher AM, Forsythe J, Nixon M, Carter RN, et al. Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab. 2016;24:130–41.
Spadaro O, Camell CD, Bosurgi L, Nguyen KY, Youm YH, Rothlin CV, et al. IGF1 shapes macrophage activation in response to immunometabolic challenge. Cell Rep. 2017;19:225–34.
Moyes KM, Graugnard DE, Khan MJ, Mukesh M, Loor JJ. Postpartal immunometabolic gene network expression and function in blood neutrophils are altered in response to prepartal energy intake and postpartal intramammary inflammatory challenge. J Dairy Sci. 2014;97:2165–77.
Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5.
del Rincón I, Polak JF, O’Leary DH, Battafarano DF, Erikson JM, Restrepo JF, et al. Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann Rheum Dis. 2015;74:1118–23.
Funding
This work was supported by Fujian Natural Science Foundation (No. 2021J02036). These funding sources played key supportive role for sample collection, molecular analysis of patient samples, bioinformatics analysis.
Author information
Authors and Affiliations
Contributions
Q-CL planned the project. Q-CL, and Q-QC conceived of and designed the study. Q-CL, X-TL, Y-JG, Y-FL and X-XG performed the sample collection. Y-ZL, Q-CL, and Y-FL performed the expression analysis. Q-CL, and Y-FL analyzed the data and drafted the manuscript. All authors reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The data collection scheme and research carried out were approved by the Medical ethics committee of the First Affiliated Hospital of Fujian Medical University. The protocol was approved by the Medical ethics committee of the First Affiliated Hospital of Fujian Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Q., Li, Y., Guo, X. et al. Selective deficiency of UCP-1 and adropin may lead to different subtypes of anti-neutrophil cytoplasmic antibody-associated vasculitis. Genes Immun 24, 39–45 (2023). https://doi.org/10.1038/s41435-023-00195-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-023-00195-x