Abstract
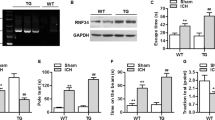
Transient receptor potential mucolipin-1 (TRPML1) is the most abundantly and widely expressed channel protein in the TRP family. While numerous studies have been conducted involving many aspects of TRPML1, such as its role in cell biology, oncology, and neurodegenerative diseases, there are limited reports about what role it plays in intracerebral hemorrhage (ICH)-induced secondary brain injury (SBI). Here we examined the function of TRPML1 in ICH-induced SBI. The caudal arterial blood of rats was injected into the caudate nucleus of basal ganglia to establish an experimental ICH model. We observed that lentivirus downregulated the expression level of TRPML1 and chemical agonist promoted the enzyme activity of TRPML1. The results indicated that the protein levels of TRPML1 in brain tissues increased 24 h after ICH. These results suggested that downregulated TRPML1 could significantly reduce inflammatory cytokines, and ICH induced the production of LDH and ROS. Furthermore, TRPML1 knockout relieved ICH-induced neuronal cell death and degeneration, and declines in learning and memory after ICH could be improved by downregulating the expression of TRPML1. In addition, chemical agonist-expressed TRPML1 showed the opposite effect and exacerbated SBI after ICH. In summary, this study demonstrated that TRPML1 contributed to brain injury after ICH, and downregulating TRPML1 could improve ICH-induced SBI, suggesting a potential target for ICH therapy.






Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the confidential policy of our hospital but are available from the corresponding author on reasonable request.
References
Baker, W. B., Balu, R., He, L., Kavuri, V. C., Busch, D. R., Amendolia, O., et al. (2019). Continuous non-invasive optical monitoring of cerebral blood flow and oxidative metabolism after acute brain injury. Journal of Cerebral Blood Flow and Metabolism, 39(8), 1469–1485. https://doi.org/10.1177/0271678x19846657
Bassi, M. T., Manzoni, M., Monti, E., Pizzo, M. T., Ballabio, A., & Borsani, G. (2000). Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. American Journal of Human Genetics, 67(5), 1110–1120. https://doi.org/10.1016/s0002-9297(07)62941-3
Bobinger, T., Burkardt, P., Huttner, H. B., & Manaenko, A. (2018). Programmed cell death after intracerebral hemorrhage. Current Neuropharmacology, 16(9), 1267–1281. https://doi.org/10.2174/1570159x15666170602112851
Bogorad, M., DeStefano, J., Linville, R., Wong, A., & Searson, P. (2019). Cerebrovascular plasticity: Processes that lead to changes in the architecture of brain microvessels. Journal of Cerebral Blood Flow & Metabolism, 39(8), 1413–1432. https://doi.org/10.1177/0271678x19855875
Carrillo-Jimenez, A., Deniz, Ö., Niklison-Chirou, M. V., Ruiz, R., Bezerra-Salomão, K., Stratoulias, V., Amouroux, R., Yip, P. K., Vilalta, A., Cheray, M., & Scott-Egerton, A. M. (2019). TET2 regulates the neuroinflammatory response in microglia. Cell Reports, 29(3), 697-713.e698. https://doi.org/10.1016/j.celrep.2019.09.013
Chen, J., Li, Y., Wang, L., Zhang, Z., Lu, D., Lu, M., & Chopp, M. (2001). Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke, 32(4), 1005–1011. https://doi.org/10.1161/01.str.32.4.1005
Cheng, X., Shen, D., Samie, M., & Xu, H. (2010). Mucolipins: Intracellular TRPML1-3 channels. FEBS Letters, 584(10), 2013–2021. https://doi.org/10.1016/j.febslet.2009.12.056
Cordonnier, C., Demchuk, A., Ziai, W., & Anderson, C. S. (2018). Intracerebral haemorrhage: Current approaches to acute management. Lancet, 392(10154), 1257–1268. https://doi.org/10.1016/s0140-6736(18)31878-6
Di Paola, S., Scotto-Rosato, A., & Medina, D. L. (2018). TRPML1: The Ca((2+))retaker of the lysosome. Cell Calcium, 69, 112–121. https://doi.org/10.1016/j.ceca.2017.06.006
Fliniaux, I., Germain, E., Farfariello, V., & Prevarskaya, N. (2018). TRPs and Ca(2+) in cell death and survival. Cell Calcium, 69, 4–18. https://doi.org/10.1016/j.ceca.2017.07.002
Forman, R., Slota, K., Ahmad, F., Garg, R., John, S., Da Silva, I., & Koffman, L. (2020). Intracerebral hemorrhage outcomes in the very elderly. Journal of Stroke and Cerebrovascular Diseases, 29(5), 104695. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.104695
Fumoto, T., Naraoka, M., Katagai, T., Li, Y., Shimamura, N., & Ohkuma, H. (2019). The role of oxidative stress in microvascular disturbances after experimental subarachnoid hemorrhage. Translational Stroke Research, 10(6), 684–694. https://doi.org/10.1007/s12975-018-0685-0
Hu, Z. D., Yan, J., Cao, K. Y., Yin, Z. Q., Xin, W. W., & Zhang, M. F. (2019). MCOLN1 promotes proliferation and predicts poor survival of patients with pancreatic ductal adenocarcinoma. Disease Markers, 2019, 9436047. https://doi.org/10.1155/2019/9436047
Jung, J., Cho, K. J., Naji, A. K., Clemons, K. N., Wong, C. O., Villanueva, M. S., Gregory, S., Karagas, N. E., Tan, L., Liang, H., Rousseau, M. A., Tomasevich, K. M., Sikora, A. G., Levental, I., van der Hoeven, D., Zhou, Y., Hancock, J. F., & Venkatachalam, K. (2019). HRAS-driven cancer cells are vulnerable to TRPML1 inhibition. EMBO Rep. https://doi.org/10.1552/embr.201846685
Ke, K., Rui, Y., Li, L., Zheng, H., Xu, W., Tan, X., Cao, J., Wu, X., Cui, G., & Cao, M. (2014). Upregulation of EHD2 after intracerebral hemorrhage in adult rats. Journal of Molecular Neuroscience, 54(2), 171–180. https://doi.org/10.1007/s12031-014-0271-1
Keep, R. F., Hua, Y., & Xi, G. (2012). Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurology, 11(8), 720–731. https://doi.org/10.1016/s1474-4422(12)70104-7
Klomparens, K., & Ding, Y. (2020). Updates on the association of brain injury and Alzheimer’s disease. Brain Circ, 6(2), 65–69. https://doi.org/10.4103/bc.bc_18_20
Lan, X., Han, X., Liu, X., & Wang, J. (2019). Inflammatory responses after intracerebral hemorrhage: From cellular function to therapeutic targets. Journal of Cerebral Blood Flow and Metabolism, 39(1), 184–186. https://doi.org/10.1177/0271678x18805675
Lattanzi, S., Cagnetti, C., Provinciali, L., & Silvestrini, M. (2017). How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovascular Diseases, 43(5–6), 207–213. https://doi.org/10.1159/000462986
Li, R. J., Xu, J., Fu, C., Zhang, J., Zheng, Y. G., Jia, H., & Liu, J. O. (2016). Regulation of mTORC1 by lysosomal calcium and calmodulin. Elife. https://doi.org/10.7554/eLife.19360
Li, X., Garrity, A. G., & Xu, H. (2013). Regulation of membrane trafficking by signalling on endosomal and lysosomal membranes. Journal of Physiology, 591(18), 4389–4401. https://doi.org/10.1113/jphysiol.2013.258301
Lindsay, M. P., Norrving, B., Sacco, R. L., Brainin, M., Hacke, W., Martins, S., Pandian, J., & Feigin, V. (2019). World stroke organization (WSO): Global stroke fact sheet 2019. International Journal of Stroke, 14(8), 806–817. https://doi.org/10.1177/1747493019881353
Liu, H., Hua, Y., Keep, R. F., & Xi, G. (2019a). Brain ceruloplasmin expression after experimental intracerebral hemorrhage and protection against iron-induced brain injury. Translational Stroke Research, 10(1), 112–119. https://doi.org/10.1007/s12975-018-0669-0
Liu, Y., Ma, C., Li, H., Shen, H., Li, X., Fu, X., Wu, J., & Chen, G. (2019b). Nogo-A/Pir-B/TrkB signaling pathway activation inhibits neuronal survival and axonal regeneration after experimental intracerebral hemorrhage in rats. Journal of Molecular Neuroscience, 69(3), 360–370. https://doi.org/10.1007/s12031-019-01365-1
Liu, Z. H., Liu, C. H., Tu, P. H., Yip, P. K., Chen, C. C., Wang, Y. C., Chen, N. Y., & Lin, Y. S. (2020). Prior antiplatelet therapy, excluding phosphodiesterase inhibitor is associated with poor outcome in patients with spontaneous intracerebral haemorrhage. Translational Stroke Research, 11(2), 185–194. https://doi.org/10.1007/s12975-019-00722-x
Medina, D. L., Di Paola, S., Peluso, I., Armani, A., Stefani, D., Venditti, R., Montefusco, S., Scotto-Rosato, A., Prezioso, C., Forrester, A., & Settembre, C. (2015). Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nature Cell Biology, 17(3), 288–299. https://doi.org/10.1038/ncb3114
Nilius, B., & Owsianik, G. (2011). The transient receptor potential family of ion channels. Genome Biology, 12(3), 218. https://doi.org/10.1186/gb-2011-12-3-218
Pu, H., Jiang, X., Hu, X., Xia, J., Hong, D., Zhang, W., Gao, Y., Chen, J., & Shi, Y. (2016). Delayed docosahexaenoic acid treatment combined with dietary supplementation of omega-3 fatty acids promotes long-term neurovascular restoration after ischemic stroke. Translational Stroke Research, 7(6), 521–534. https://doi.org/10.1007/s12975-016-0498-y
Rupprecht, S., Finn, S., Hoyer, D., Guenther, A., Witte, O. W., Schultze, T., & Schwab, M. (2020). Association between systemic inflammation, carotid arteriosclerosis, and autonomic dysfunction. Translational Stroke Research, 11(1), 50–59. https://doi.org/10.1007/s12975-019-00706-x
Rutkai, I., Merdzo, I., Wunnava, S. V., Curtin, G. T., Katakam, P. V., & Busija, D. W. (2019). Cerebrovascular function and mitochondrial bioenergetics after ischemia-reperfusion in male rats. Journal of Cerebral Blood Flow and Metabolism, 39(6), 1056–1068. https://doi.org/10.1177/0271678x17745028
Saand, A. R., Yu, F., Chen, J., & Chou, S. H. (2019). Systemic inflammation in hemorrhagic strokes—A novel neurological sign and therapeutic target? Journal of Cerebral Blood Flow and Metabolism, 39(6), 959–988. https://doi.org/10.1177/0271678x19841443
Sergeant, G. P., Hollywood, M. A., & Thornbury, K. D. (2020). Igniting Ca(2+) sparks with TRPML1. Proc Natl Acad Sci U S A, 117(52), 32836–32838. https://doi.org/10.1073/pnas.2022896117
Settembre, C., Di Malta, C., Polito, V. A., Garcia Arencibia, M., Vetrini, F., Erdin, S., Erdin, S. U., Huynh, T., Medina, D., Colella, P., & Sardiello, M. (2011). TFEB links autophagy to lysosomal biogenesis. Science, 332(6036), 1429–1433. https://doi.org/10.1126/science.1204592
Shen, F., Xu, X., Yu, Z., Li, H., Shen, H., Li, X., Shen, M., & Chen, G. (2021). Rbfox-1 contributes to CaMKIIα expression and intracerebral hemorrhage-induced secondary brain injury via blocking micro-RNA-124. Journal of Cerebral Blood Flow and Metabolism, 41(3), 530–545. https://doi.org/10.1177/0271678x20916860
Spix, B., Chao, Y. K., Abrahamian, C., Chen, C. C., & Grimm, C. (2020). TRPML cation channels in inflammation and immunity. Frontiers in Immunology, 11, 225. https://doi.org/10.3389/fimmu.2020.00225
Sukumari-Ramesh, S., Alleyne, C. H., Jr., & Dhandapani, K. M. (2016). The histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) confers acute neuroprotection after intracerebral hemorrhage in mice. Translational Stroke Research, 7(2), 141–148. https://doi.org/10.1007/s12975-015-0421-y
Suzuki, H. (2019). Inflammation: A good research target to improve outcomes of poor-grade subarachnoid hemorrhage. Translational Stroke Research, 10(6), 597–600. https://doi.org/10.1007/s12975-019-00713-y
Tschoe, C., Bushnell, C. D., Duncan, P. W., Alexander-Miller, M. A., & Wolfe, S. Q. (2020). Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke, 22(1), 29–46. https://doi.org/10.5853/jos.2019.02236
Turlova, E., Wong, R., Xu, B., Li, F., Du, L., Habbous, S., David Horgen, F., Fleig, A., Feng, Z.-P., & Sun, H.-S. (2021). TRPM7 mediates neuronal cell death upstream of calcium/calmodulin-dependent protein kinase II and calcineurin mechanism in neonatal hypoxic-ischemic brain injury. Translational Stroke Research, 12(1), 164–184. https://doi.org/10.1007/s12975-020-00810-3
Wang, Y., Jiang, S. W., Liu, X., Niu, L., Ge, X. L., Zhang, J. C., Wang, H. R., Fei, A. H., Gao, C. J., & Pan, S. M. (2018). Degradation of TRPML1 in neurons reduces neuron survival in transient global cerebral ischemia. Oxidative Medicine and Cellular Longevity, 2018, 4612727. https://doi.org/10.1155/2018/4612727
Wei, J., Wang, M., Jing, C., Keep, R. F., Hua, Y., & Xi, G. (2020). Multinucleated giant cells in experimental intracerebral hemorrhage. Translational Stroke Research, 11(5), 1095–1102. https://doi.org/10.1007/s12975-020-00790-4
Wu, J., He, J., Tian, X., Zhong, J., Li, H., & Sun, X. (2020). Activation of the hedgehog pathway promotes recovery of neurological function after traumatic brain injury by protecting the neurovascular unit. Translational Stroke Research, 11(4), 720–733. https://doi.org/10.1007/s12975-019-00771-2
Wu, S., Wu, B., Liu, M., Chen, Z., Wang, W., Anderson, C. S., Sandercock, P., Wang, Y., Huang, Y., Cui, L., Pu, C., Jia, J., Zhang, T., Liu, X., Zhang, S., Xie, P., Fan, D., Ji, X., Lawrence Wong, K.-S., & Wang, L. (2019). Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurology, 18(4), 394–405. https://doi.org/10.1016/s1474-4422(18)30500-3
Xia, Z., Ren, Y., Li, S., Xu, J., Wu, Y., & Cao, Z. (2021). ML-SA1 and SN-2 inhibit endocytosed viruses through regulating TRPML channel expression and activity. Antiviral Research, 195, 105193. https://doi.org/10.1016/j.antiviral.2021.105193
Xia, Z., Wang, L., Li, S., Tang, W., Sun, F., Wu, Y., Chen, N.-Y., & Lin, Y.-S. (2020). ML-SA1, a selective TRPML agonist, inhibits DENV2 and ZIKV by promoting lysosomal acidification and protease activity. Antiviral Research, 182, 104922. https://doi.org/10.1016/j.antiviral.2020.104922
Xu, H., Cao, J., Xu, J., Li, H., Shen, H., Li, X., Wang, Z., Wu, J., & Chen, G. (2019). GATA-4 regulates neuronal apoptosis after intracerebral hemorrhage via the NF-κB/Bax/caspase-3 pathway both in vivo and in vitro. Experimental Neurology, 315, 21–31. https://doi.org/10.1016/j.expneurol.2019.01.018
Zhang, J., Zhang, W., Gao, X., Zhao, Y., Chen, D., Xu, N., Pu, H., Stetler, R. A., & Gao, Y. (2019b). Preconditioning with partial caloric restriction confers long-term protection against grey and white matter injury after transient focal ischemia. Journal of Cerebral Blood Flow and Metabolism, 39(7), 1394–1409. https://doi.org/10.1177/0271678x18785480
Zhang, L., Fang, Y., Cheng, X., Lian, Y., Xu, H., Zeng, Z., & Zhu, H. (2017a). TRPML1 participates in the progression of Alzheimer’s disease by regulating the PPARγ/AMPK/Mtor signalling pathway. Cellular Physiology and Biochemistry, 43(6), 2446–2456. https://doi.org/10.1159/000484449
Zhang, P., Liu, X., Li, H., Chen, Z., Yao, X., Jin, J., & Ma, X. (2017b). TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKβ/AMPKα/mTOR pathway. Science and Reports, 7(1), 3158. https://doi.org/10.1038/s41598-017-03230-w
Zhang, P., Wang, T., Zhang, D., Zhang, Z., Yuan, S., Zhang, J., Cao, J., Li, H., Li, X., Shen, H., & Chen, G. (2019a). Exploration of MST1-mediated secondary brain injury induced by intracerebral hemorrhage in rats via hippo signaling pathway. Translational Stroke Research, 10(6), 729–743. https://doi.org/10.1007/s12975-019-00702-1
Zhang, S., Hu, Z. W., Luo, H. Y., Mao, C. Y., Tang, M. B., Li, Y. S., Song, B., Wang, Y. H., Zhang, Z. X., Zhang, Q. M., & Fan, L. Y. (2020). AAV/BBB-mediated gene transfer of CHIP attenuates brain injury following experimental intracerebral hemorrhage. Translational Stroke Research, 11(2), 296–309. https://doi.org/10.1007/s12975-019-00715-w
Funding
The National Natural Science Foundation of China (No.81830036), the Natural Science Foundation of Jiangsu Province under Grant (No. BK20220096), the Suzhou Science and Technology (No. SS2019056), Jiangsu Commission of Health (No. K2019001), Suzhou Key Medical Center (No. Szzx201501), and Suzhou Government (No. SYS2019045).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
All protocols for laboratory animals are approved by the Animal Care and Use Committee of Soochow University and implemented in accordance with the manuals of the National Institutes of Health. The ethical approval reference number is 2018-198.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, J., Li, X., Ding, J. et al. Transient Receptor Potential Mucolipin-1 Participates in Intracerebral Hemorrhage-Induced Secondary Brain Injury by Inducing Neuroinflammation and Neuronal Cell Death. Neuromol Med 25, 272–285 (2023). https://doi.org/10.1007/s12017-023-08734-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-023-08734-5