Abstract
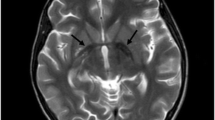
Neurodegeneration with brain iron accumulation (NBIA) is an umbrella term encompassing various inherited neurological disorders characterised by abnormal iron accumulation in basal ganglia. We aimed to study the clinical, radiological and molecular spectrum of disorders with NBIA. All molecular-proven cases of NBIA presented in the last 5 years at 2 tertiary care genetic centres were compiled. Demographic details and clinical and neuroimaging findings were collated. We describe 27 individuals from 20 unrelated Indian families with causative variants in 5 NBIA-associated genes. PLA2G6-associated neurodegeneration (PLAN) was the most common, observed in 13 individuals from 9 families. They mainly presented in infancy with neuroregression and hypotonia. A recurrent pathogenic variant in COASY was observed in two neonates with prenatal-onset severe neurodegeneration. Pathogenic bi-allelic variants in PANK2, FA2H and C19ORF12 genes were observed in the rest, and these individuals presented in late childhood and adolescence with gait abnormalities and extrapyramidal symptoms. No intrafamilial and interfamilial variability were observed. Iron deposition on neuroimaging was seen in only 6/17 (35.3%) patients. A total of 22 causative variants across 5 genes were detected including a multiexonic duplication in PLA2G6. The variants c.1799G > A and c.2370 T > G in PLA2G6 were observed in three unrelated families. In silico assessments of 8 amongst 9 novel variants were also performed. We present a comprehensive compilation of the phenotypic and genotypic spectrum of various subtypes of NBIA from the Indian subcontinent. Clinical presentation of NBIAs is varied and not restricted to extrapyramidal symptoms or iron accumulation on neuroimaging.


Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Gregory A, Polster BJ, Hayflick SJ (2009) Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet 46(2):73–80. https://doi.org/10.1136/jmg.2008.061929
Hogarth P (2015) Neurodegeneration with brain iron accumulation: diagnosis and management. J Mov Disord 8(1):1–13. https://doi.org/10.14802/jmd.14034
Richards R, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Mishra R, Kulshreshtha S, Mandal K, et al (2022). COASY related pontocerebellar hypoplasia type 12: a common Indian mutation with the expansion of the phenotypic spectrum. Am J Med Genet A May 2. https://doi.org/10.1002/ajmg.a.62768
Kausthubham N, Shukla A, Gupta N et al (2021) A data set of variants derived from 1455 clinical and research exomes is efficient in variant prioritization for early-onset monogenic disorders in Indians. Hum Mutat 42(4):e15–e61. https://doi.org/10.1002/humu.24172
Schneider SA, Dusek P, Hardy J, Westenberger A, Jankovic J, Bhatia KP (2013) Genetics and pathophysiology of neurodegeneration with brain iron accumulation (NBIA). Curr Neuropharmacol 11(1):59–79. https://doi.org/10.2174/157015913804999469
Wilson JL, Gregory A, Kurian MA, BPAN Guideline Contributing Author Group, Hogarth P, Hayflick SJ et al (2021) Consensus clinical management guideline for beta-propeller protein-associated neurodegeneration. Dev Med Child Neurol 63(12):1402–1409. https://doi.org/10.1111/dmcn.14980
Phadke SR, Srivastava P, Sharma P, Rai A, Masih S (2021) Homozygosity stretches around homozygous mutations in autosomal recessive disorders: patients from nonconsanguineous Indian families. J Genet 100:2
Kurian MA, Morgan NV, MacPherson L et al (2008) Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN). Neurology 70(18):1623–1629. https://doi.org/10.1212/01.wnl.0000310986.48286.8e
Romani M, Kraoua I, Micalizzi A et al (2015) Infantile and childhood onset PLA2G6-associated neurodegeneration in a large North African cohort. Eur J Neurol 22(1):178–186. https://doi.org/10.1111/ene.12552
Guo YP, Tang BS, Guo JF (2018) PLA2G6-associated neurodegeneration (PLAN): review of clinical phenotypes and Genotypes. Front Neurol 9:1100. https://doi.org/10.3389/fneur.2018.01100
Altuame FD, Foskett G, Atwal PS et al (2020) The natural history of infantile neuroaxonal dystrophy. Orphanet J Rare Dis 15(1):109. https://doi.org/10.1186/s13023-020-01355-2
Illingworth MA, Meyer E, Chong WK et al (2014) PLA2G6-associated neurodegeneration (PLAN): further expansion of the clinical, radiological and mutation spectrum associated with infantile and atypical childhood-onset disease. Mol Genet Metab 112(2):183–189. https://doi.org/10.1016/j.ymgme.2014.03.008
Jain S, Bhasin H, Romani M, Valente EM, Sharma S (2019) Atypical childhood-onset neuroaxonal dystrophy in an Indian girl. J Pediatr Neurosci 14(2):90–93. https://doi.org/10.4103/jpn.JPN_91_18
Kapoor S, Shah MH, Singh N et al (2016) Genetic analysis of PLA2G6 in 22 Indian families with infantile neuroaxonal dystrophy, atypical late-onset neuroaxonal dystrophy and dystonia Parkinsonism complex. PLoS One 11(5):e0155605. https://doi.org/10.1371/journal.pone.0155605
Bhardwaj NK, Gowda VK, Saini J, Sardesai AV, Santhoshkumar R, Mahadevan A (2021) Neurodegeneration with brain iron accumulation: Characterization of clinical, radiological, and genetic features of pediatric patients from Southern India. Brain Dev 43(10):1013–1022. https://doi.org/10.1016/j.braindev.2021.06.010
Crompton D, Rehal PK, MacPherson L et al (2010) Multiplex ligation-dependent probe amplification (MLPA) analysis is an effective tool for the detection of novel intragenic PLA2G6 mutations: implications for molecular diagnosis. Mol Genet Metab 100(2):207–212. https://doi.org/10.1016/j.ymgme.2010.02.009
Akcakaya NH, Iseri SU et al (2017) Clinical and genetic features of PKAN patients in a tertiary centre in Turkey. Clin Neurol Neurosurg 154:34–42. https://doi.org/10.1016/j.clineuro.2017.01.011
Morales-Briceño H, Chacón-Camacho OF, Pérez-González EA et al (2015) Clinical, imaging, and molecular findings in a sample of Mexican families with pantothenate kinase-associated neurodegeneration. Clin Genet 87(3):259–265. https://doi.org/10.1111/cge.12400
Hartig MB, Hörtnagel K, Garavaglia B et al (2006) Genotypic and phenotypic spectrum of PANK2 mutations in patients with neurodegeneration with brain iron accumulation. Ann Neurol 59(2):248–256. https://doi.org/10.1002/ana.20771
Delgado RF, Sanchez PR, Speckter H et al (2012) Missense PANK2 mutation without “eye of the tiger” sign: MR findings in a large group of patients with pantothenate kinase-associated neurodegeneration (PKAN). J Magn Reson Imaging 35(4):788–794. https://doi.org/10.1002/jmri.22884
Chang CL, Lin CM (2011) Eye-of-the-tiger sign is not pathognomonic of pantothenate kinase-associated neurodegeneration in adult cases. Brain Behav 1(1):55–56. https://doi.org/10.1002/brb3.8
Rattay TW, Lindig T, Baets J et al (2019) FAHN/SPG35: a narrow phenotypic spectrum across disease classifications. Brain 142(6):1561–1572. https://doi.org/10.1093/brain/awz102
Jain V, Bijarnia-Mahay S, Ramprasad VL, Saxena R, Verma IC (2018) Fatty acid hydroxylase- associated neurodegeneration - a rare case of neurodegeneration with brain iron accumulation (NBIA). Genet Clin 11:6–9
Hartig M, Prokisch H, Meitinger T, Klopstock T (2013) Mitochondrial membrane protein-associated neurodegeneration (MPAN). Int Rev Neurobiol 110:73–84. https://doi.org/10.1016/B978-0-12-410502-7.00004-1
Incecik F, Herguner OM, Bisgin A (2020) Mitochondrial membrane protein-associated neurodegeneration: a case series of six children. Ann Indian Acad Neurol 23(6):802–804. https://doi.org/10.4103/aian.AIAN_268_19
Olgiati S, Doğu O, Tufekcioglu ZETAL (2017) The p.Thr11Met mutation in c19orf12 is frequent among adult Turkish patients with MPAN. Parkinsonism Relat Disord 39:64–70. https://doi.org/10.1016/j.parkreldis.2017.03.012
Hogarth P, Gregory A, Kruer MC et al (2013) New NBIA subtype: genetic, clinical, pathologic, and radiographic features of MPAN. Neurology 80:268–275. https://doi.org/10.1212/WNL.0b013e31827e07be
Gregory A, Lotia M, Jeong SY et al (2019) Autosomal dominant mitochondrial membrane protein-associated neurodegeneration (MPAN). Mol Genet Genomic Med 7(7):e00736. https://doi.org/10.1002/mgg3.736
Skowronska M, Kmiec T, Jurkiewicz E, Malczyk K, Kurkowska-Jastrzebska I, Czlonkowska A (2017) Evolution and novel radiological changes of neurodegeneration associated with mutations in C19orf12. Parkinsonism Relat Disord 39:71–76. https://doi.org/10.1016/j.parkreldis.2017.03.013
Van Dijk T, Ferdinandusse S, Ruiter JPN et al (2018) Biallelic loss of function variants in COASY cause prenatal onset pontocerebellar hypoplasia, microcephaly, and arthrogryposis. Eur J Hum Genet 26(12):1752–1758. https://doi.org/10.1038/s41431-018-0233-0
Dusi S, Valletta L, Haack TB et al (2014) Exome sequence reveals mutations in CoA synthase as a cause of neurodegeneration with brain iron accumulation. Am J Hum Genet 94(1):11–22. https://doi.org/10.1016/j.ajhg.2013.11.008
Annesi G, Gagliardi M, Iannello G, Quattrone A, Iannello G, Quattrone A (2016) Mutational analysis of COASY in an Italian patient with NBIA. Parkinsonism Relat Disord 28:150–151. https://doi.org/10.1016/j.parkreldis.2016.03.011
Evers C, Seitz A, Assmann B et al (2017) Diagnosis of CoPAN by whole exome sequencing: waking up a sleeping tiger’s eye. Am J Med Genet A 173(7):1878–1886. https://doi.org/10.1002/ajmg.a.38252
Haack TB, Ignatius E, Calvo-Garrido J et al (2016) Absence of the autophagy adaptor SQSTM1/p62 causes childhood-onset neurodegeneration with ataxia, dystonia, and gaze palsy. Am J Hum Genet 99(3):735–743. https://doi.org/10.1016/j.ajhg.2016.06.026
Funding
We received support from the following: “The Department of Biotechnology, New Delhi, India” (Grant number: BT/PR26428/MED/12/783/2017), “Indian Council of Medical Research, New Delhi, India” (Grant Number: 33/9/2019-TF/Rare/BMS, 33/2/2019-TF/Rare/BMS) and “National Institutes of Health,NIH, USA” (Grant Number: 1R01HD093570-01A1).
Author information
Authors and Affiliations
Contributions
• AM: 1A,1B,1C,3B,2C.
• HS: Writing -2A,2B,3B.
• SS,AS,PM,LP: 2C
• MP: 2A,2B
• DR: 2A
• AS: 2C,3B
• KM,DS,SS: 1C,2C,3B
• SP: 1A,1B,1C,3B
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the institutional ethics committees of Sanjay Gandhi Postgraduate Institute of Medical Sciences and Kasturba Medical College. Informed written consent was obtained from the parents of study participants. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sait, H., Srivastava, S., Pandey, M. et al. Neurodegeneration with brain iron accumulation: a case series highlighting phenotypic and genotypic diversity in 20 Indian families. Neurogenetics 24, 113–127 (2023). https://doi.org/10.1007/s10048-023-00712-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-023-00712-0