Abstract
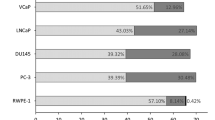
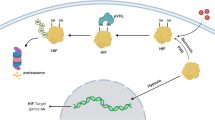
A low-oxygen (hypoxia) tumor microenvironment can facilitate chemotherapy and radiation therapy resistance in tumors and is associated with a poor prognosis. Hypoxia also affects PCa (prostate cancer) phenotype transformation and causes therapeutic resistance. Although O-glycans are known to be involved in the malignancy of various cancers under hypoxia, the expression and function of O-glycans in PCa are not well understood. In this study, the saccharide primer method was employed to analyze O-glycan expression in PCa cells. Results showed that the expression of sTn antigens was increased in PCa cells under hypoxia. Furthermore, it was found that ST6GalNAc1, the sTn antigen synthase gene, was involved in the migration–proliferation dichotomy and drug resistance in PCa cells under hypoxia. The results of this study will contribute to the development of novel diagnostic markers and drug targets for PCa under hypoxia.




Similar content being viewed by others
Data availability
All data generated or analyzed in this study are included in this article and its supplemental information files.
Abbreviations
- TME:
-
Tumor microenvironment
- HIF-1α:
-
Hypoxia-inducible factor α
- PCa:
-
Prostate cancer
- CRPC:
-
Castration-resistant prostate cancer
- Asn:
-
Asparagine
- Thr:
-
Threonine
- LC-MS:
-
Liquid chromatography-mass spectrometry
- GalNAc:
-
N-Acetylgalactosamine
- GlcNAc:
-
N-Acetylglucosamine
- sTn:
-
Sialyl-Tn
- C1GalT1:
-
glycoprotein-N-acetylgalactosamine 3-β-galactosyltransferase
- GCNT3:
-
β-1,6-N-Acetylglucosaminyltransferase gene 3
- ST6GalNAc1:
-
ST6 N-acetylgalactosaminide α-2,6-sialyltransferase 1
- B3GALT5:
-
β-1,3-Galactosyltransferase 5
- B4GALT1/5:
-
β-1,4-Galactosyltransferase 1/5
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- EGFR:
-
Epidermal growth factor receptor
- MDR:
-
Multidrug resistance
References
De Palma, M., Biziato, D., Petrova, T.V.: Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017). https://doi.org/10.1038/nrc.2017.51
Lim, B., Woodward, W.A., Wang, X., Reuben, J.M., Ueno, N.T.: Inflammatory breast cancer biology: the tumour microenvironment is key. Nat. Rev. Cancer 18, 485–499 (2018). https://doi.org/10.1038/s41568-018-0010-y
Fernández, J.P., Luddy, K.A., Harmon, C., O’Farrelly, C.: Hepatic tumor microenvironments and effects on NK cell phenotype and function. Int. J. Mol. Sci 20, 4131 (2019). https://doi.org/10.3390/ijms20174131
Bejarano, L., Jordāo, M.J.C., Joyce, J.A.: Therapeutic targeting of the tumor microenvironment. Cancer Discov 4, 933–959 (2021). https://doi.org/10.1158/2159-8290.CD-20-1808
Wei, J., Chen, Z., Hu, M., He, Z., Jiang, D., Long, J., Du, H.: Characterizing intercellular communication of pan-cancer reveals SPP1+ tumor-associated macrophage expanded in hypoxia and promoting cancer maligancy through single-cell RNA-seq data. Front. Cell. Dev. Biol 9, 749210 (2021). https://doi.org/10.3389/fcell.2021.749210
Greville, G., Llop, E., Huang, C., Creagh-Flynn, J., Pfister, S., O’Flaherty, R., Madden, S.F., Peracaula, R., Rudd, P.M., McCann, A., Saldova, R.: Hypoxia alters epigenetic and N-glycosylation profiles of ovarian and breast cancer cell lines in-vitro Front. Oncol 10, 1218 (2020). https://doi.org/10.3389/fonc.2020.01218
Wilson, W.R., Hay, M.P.: Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 (2011). https://doi.org/10.1038/nrc3064
Arriagada, C., Silva, P., Torres, V.A.: Role of glycosylation in hypoxia-driven cell migration and invasion. Cell. Adhes. Migr 13, 13–22 (2019). https://doi.org/10.1080/19336918.2018.1491234
Ma, S., Zhao, Y., Lee, W.C., Ong, L., Lee, P.L., Jiang, Z., Oguz, G., Niu, Z., Liu, M., Goh, J.Y., Wang, W., Bustos, M.A., Ehmsen, S., Ramasamy, A., Hoon, D.S.B., Ditzel, H.J., Tan, E.Y., Chen, Q., Yu, Q.: Hypoxia induces HIF1α-dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti-PD-1 immunotherapy. Nat. Commun 13, 4118 (2022). https://doi.org/10.1038/s41467-022-31764-9
Peng, F., Wang, J.H., Fan, W.J., Meng, Y.T., Li, M.M., Li, T.T., Cui, B., Wang, H.F., Zhao, Y., An, F., Guo, T., Liu, X.F., Zhang, L., Lv, L., Lv, D.K., Xu, L.Z., Xie, J.J., Lin, W.X., Lam, E.W.F., Xu, J., Liu, Q.: Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene 37, 1062–1074 (2018). https://doi.org/10.1038/onc.2017.368
Zhang, Y., Hong, Y., Wang, D., Duan, L., Liu, Y., Li, L., Liu, D., Zhuang, K., Chaoxin, W., Zheng, G., Chunyong, H., Guoyan, L.: Hsa_circ_0076305 induces migration-proliferation dichotomy in gastric cancer. Oncol. Lett 21, 220 (2021). https://doi.org/10.3892/ol.2021.12481
Bhandari, D., Lopez-Sanchez, I., To, A., Lo, I.C., Aznar, N., Leyme, A., Gupta, V., Niesman, I., Maddox, A.L., Garcia-Marcos, M., Farquhar, M.G., Ghosh, P.: Cyclin-dependent kinase 5 activates guanine nucleotide exchange factor GIV/Girdin to orchestrate migration-proliferation dichotomy. Proc. Natl. Acad. Sci. U. S. A. 112, E4874–E4883 (2015). https://doi.org/10.1073/pnas.1514157112
Chen, C., Enomoto, A., Weng, L., Taki, T., Shiraki, Y., Mii, S., Ichihara, R., Kanda, M., Koike, M., Kodera, Y., Takahashi, M.: Complex roles of the actin-binding protein Girdin/GIV in DNA damage-induced apoptosis of cancer cells. Cancer Sci 111, 4303–4317 (2020). https://doi.org/10.1111/cas.14637
Ghosh, P., Beas, A.O., Bornheimer, S.J., Garcia-Marcos, M., Forry, E.P., Johannson, C., Ear, J., Jung, B.H., Cabrera, B., Carethers, J.M., Farquhar, M.G.: A Gαi-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol. Biol. Cell 21, 2338–2354 (2010). https://doi.org/10.1091/mbc.E10-01-0028
Wang, S.D., Rath, P., Lal, B., Richard, J.P., Li, Y., Goodwin, C.R., Laterra, J., Xia, S.: EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene 31, 5132–5143 (2012). https://doi.org/10.1038/onc.2012.16
Chen, J., Sun, Y., Xu, X., Wang, D., He, J., Zhou, H., Lu, Y., Zeng, J., Du, F., Gong, A., Xu, M.: YTH domain family 2 orchestrates epithelial- mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell. Cycle 16, 2259–2271 (2017). https://doi.org/10.1080/15384101.2017.1380125
Stein, M.N., Patel, N., Bershadskiy, A., Sokoloff, A., Singer, E.A.: Androgen synthesis inhibitors in the treatment of castration-resistant prostate cancer. Asian J. Androl 16, 387–400 (2014). https://doi.org/10.4103/1008-682X.129133
Hochachka, P.W., Rupert, J.L., Goldenberg, L., Gleave, M., Kozlowski, P.: Going malignant: the hypoxia-cancer connection in the prostate. BioEssays 24, 749–757 (2002). https://doi.org/10.1002/bies.10131
Movsas, B., Chapman, J.D., Hanlon, A.L., Horwitz, E.M., Pinover, W.H., Greenberg, R.E., Stobbe, C., Hanks, G.E.: Hypoxia in human prostate carcinoma: an Eppendorf PO2 study. Am. J. Clin. Oncol 24, 458–461 (2001). https://doi.org/10.1097/00000421-200110000-00009
Yasumizu, Y., Hongo, H., Kosaka, T., Mikami, S., Nishimoto, K., Kikuchi, E., Oya, M.: PKM2 under hypoxic environment causes resistance to mTOR inhibitor in human castration resistant prostate cancer. Oncotarget 9, 27698–27707 (2018). https://doi.org/10.18632/oncotarget.25498
Alqawi, O., Moghaddas, M., Singh, G.: Effects of geldanamycin on HIF-1α mediated angiogenesis and invasion in prostate cancer cells. Prostate Cancer Prostatic Dis 9, 126–135 (2006). https://doi.org/10.1038/sj.pcan.4500852
Fraga, A., Ribeiro, R., Príncipe, P., Lopes, C., Medeiros, R.: Hypoxia and prostate cancer aggressiveness: a tale with many endings. Clin. Genitourin. Cancer 4, 295–301 (2015). https://doi.org/10.1016/j.clgc.2015.03.006
Pearce, O.M.T.: Cancer glycan epitopes: biosynthesis, structure and function. Glycobiology 28, 670–696 (2018). https://doi.org/10.1093/glycob/cwy023
Chernykh, A., Kawahara, R., Thaysen-Andersen, M.: Towards structure-focused glycoproteomics. Biochem. Soc. Trans 49, 161–186 (2021). https://doi.org/10.1042/BST20200222
Ho, W.L., Hsu, W.M., Huang, M.C., Kadomatsu, K., Nakagawara, A.: Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma. J. Hematol. Oncol 9, 100 (2016). https://doi.org/10.1186/s13045-016-0334-6
Potapenko, I.O., Haakensen, V.D., Lüders, T., Helland, Ã, Bukholm, I., Sørlie, T., Kristensen, V.N., Lingjærde, O.C., Børresen-Dale, A.L.: Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol. Oncol 4, 98–118 (2010). https://doi.org/10.1016/j.molonc.2009.12.001
Hollingsworth, M.A., Swanson, B.J.: Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 4, 45–60 (2004). https://doi.org/10.1038/nrc1251
Wandall, H.H., Nielsen, M.A.I., King-Smith, S., de Haan, N., Bagdonaite, I.: Global functions of O-glycosylation: promises and challenges in O-glycobiology. FEBS J 288, 7183–7212 (2021). https://doi.org/10.1111/febs.16148
Tan, Z., Wang, C., Li, X., Guan, F.: Bisecting N-acetylglucosamine structures inhibit hypoxia-induced epithelial-mesenchymal transition in breast cancer cells. Front. Psychiatry 9, 210 (2018). https://doi.org/10.3389/fphys.2018.00210
Koike, T., Kimura, N., Miyazaki, K., Yabuta, T., Kumamoto, K., Takenoshita, S., Chen, J., Kobayashi, M., Hosokawa, M., Taniguchi, A., Kojima, T., Ishida, N., Kawakita, M., Yamamoto, H., Takematsu, H., Suzuki, A., Kozutsumi, Y., Kannagi, R.: Erratum: Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc. Natl. Acad. Sci. U. S. A. 21, 8132-7 (2004). https://doi.org/10.1073/pnas.0402088101
Miura, Y., Arai, T., Yamagata, T.: Synthesis of amphiphilic lactosides that possess a lactosylceramide-mimicking N-acyl structure: alternative universal substrates for endo-type glycosylceramidases. Carbohydr. Res 289, 193–199 (1996). https://doi.org/10.1016/0008-6215(96)00132-2
Miura, Y., Yamagata, T.: Glycosylation of lactosylceramide analogs in animal cells: amphipathic disaccharide primers for glycosphingolipid synthesis. Biochem. Biophys. Res. Commun 241, 698–703 (1997). https://doi.org/10.1006/bbrc.1997.7876
Nakajima, H., Miura, Y., Yamagata, T.: Glycosylation of amphipathic lactoside primers with consequent inhibition of endogenous glycosphingolipid synthesis. J. Biochem 124, 148–156 (1998). https://doi.org/10.1093/oxfordjournals.jbchem.a022073
Sato, T., Takashiba, M., Hayashi, R., Zhu, X., Yamagata, T.: Glycosylation of dodecyl 2-acetamido-2-deoxy-β-D-glucopyranoside and dodecyl β-D-galactopyranosyl-(1→4)-2-acetamido-2-deoxy-β-D-glucopyranoside as saccharide primers in cells. Carbohydr. Res. 343, 831–838 (2008). https://doi.org/10.1016/j.carres.2008.01.022
Zhu, X., Hatanaka, K., Yamagata, T., Sato, T.: Structural analysis of glycosphingolipid analogues obtained by the saccharide primer method using CE-ESI-MS. Electrophoresis 30, 3519–3526 (2009). https://doi.org/10.1002/elps.200800719
Otsuka, Y., Sato, T.: Saccharide primers comprising xylosyl-serine primed phosphorylated oligosaccharides act as intermediates in glycosaminoglycan biosynthesis. ACS Omega 2, 3110–3122 (2017). https://doi.org/10.1021/acsomega.7b00073
Otsuka, Y., Sato, T.: Comparative quantification method for glycosylated products elongated on β-xylosides using a stable isotope-labeled saccharide primer. Anal. Chem 90, 5201–5208 (2018). https://doi.org/10.1021/acs.analchem.7b05438
Sakura, R., Nagai, K., Yagi, Y., Takahashi, Y., Ide, Y., Yagi, Y.: In vitro synthesis of mucin-type O-glycans using saccharide primers comprising GalNAc-Ser and GalNAc-Thr residues. Carbohydr. Res 511, 108495 (2022). https://doi.org/10.1016/j.carres.2021.108495
Yamamoto, D., Sasaki, K., Kosaka, T., Oya, M., Sato, T.: Functional analysis of GCNT3 for cell migration and EMT of castration-resistant prostate cancer cells. Glycobiology 32, 897–908 (2022). https://doi.org/10.1093/glycob/cwac044
Chang, Y.S., Chen, W.Y., Yin, J.J., Sheppard-Tillman, H., Huang, J., Liu, Y.N.: EGF receptor promotes prostate cancer bone metastasis by downregulating miR-1 and activating TWIST1. Cancer Res 75, 3077–3086 (2015). https://doi.org/10.1158/0008-5472.CAN-14-3380
Qin, H., Liu, J., Yu, M., Wang, H., Thomas, A.M., Li, S., Yan, Q., Wang, L.: FUT7 promotes the malignant transformation of follicular thyroid carcinoma through α1,3-fucosylation of EGF receptor. Exp. Cell. Res 393, 112095 (2020). https://doi.org/10.1016/j.yexcr.2020.112095
Jing, X., Yang, F., Shao, C., Wei, K., Xie, M., Shen, H., Shu, Y.: Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 18, 157 (2019). https://doi.org/10.1186/s12943-019-1089-9
Tao, J., Yang, G., Zhou, W., Qiu, J., Chen, G., Luo, W., Zhao, F., You, L., Zheng, L., Zhang, T., Zhao, Y.: Targeting hypoxic tumor microenvironment in pancreatic cancer. J. Hematol. Oncol 14, 14 (2021). https://doi.org/10.1186/s13045-020-01030-w
Marotta, D., Karar, J., Jenkins, W.T., Kumanova, M., Jenkins, K.W., Tobias, J.W., Baldwin, D., Hatzigeorgiou, A., Alexiou, P., Evans, S.M., Alarcon, R., Maity, A., Koch, C., Koumenis, C.: In vivo profiling of hypoxic gene expression in gliomas using the hypoxia marker EF5 and laser-capture microdissection. Cancer Res 71, 779–789 (2011). https://doi.org/10.1158/0008-5472.CAN-10-3061
Silva-Filho, A.F., Sena, W.L.B., Lima, L.R.A., Carvalho, L.V.N., Pereira, M.C., Santos, L.G.S., Santos, R.V.C., Tavares, L.B., Pitta, M.G.R., Rêgo, M.J.B.M.: Glycobiology modifications in intratumoral hypoxia: the breathless side of glycans interaction. Cell. Physiol. Biochem 41, 1801–1829 (2017). https://doi.org/10.1159/000471912
Sun, R., Kim, A.M.J., Lim, S.O.: Glycosylation of immune receptors in cancer. Cells 10, 1100 (2021). https://doi.org/10.3390/cells10051100
Sood, A., Ma, S., Pradeep, S., Hu, W., Zhang, D., Coleman, R.: The role of tumor microenvironment in resistance to anti-angiogenic therapy. F1000Res 7, 326 (2018). https://doi.org/10.12688/f1000research.11771.1
Croci, D.O., Cerliani, J.P., Pinto, N.A., GMorosi, L., Rabinovich, G.A.: Regulatory role of glycans in the control of hypoxia-driven angiogenesis and sensitivity to anti-angiogenic treatment. Glycobiology 24, 1283–1290 (2014). https://doi.org/10.1093/glycob/cwu083
Jones, R.B., Dorsett, K.A., Hjelmeland, A.B., Bellis, S.L.: The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1 signaling. J. Biol. Chem 293, 5659–5667 (2018). https://doi.org/10.1074/jbc.RA117.001194
Stewart, G.D., Ross, J.A., McLaren, D.B., Parker, C.C., Habib, F.K., Riddick, A.C.P.: The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int 105, 8–13 (2010). https://doi.org/10.1111/j.1464-410X.2009.08921.x
Lee, J.E., Shin, S.H., Shin, H.W., Chun, Y.S., Park, J.W.: Nuclear FGFR2 negatively regulates hypoxia-induced cell invasion in prostate cancer by interacting with HIF-1 and HIF-2. Sci. Rep 9, 3480 (2019). https://doi.org/10.1038/s41598-019-39843-6
Butterworth, K.T., McCarthy, H.O., Devlin, A., Ming, L., Robson, T., McKeown, S.R., Worthington, J.: Hypoxia selects for androgen independent LNCaP cells with a more malignant geno- and phenotype. Int. J. Cancer 123, 760–768 (2008). https://doi.org/10.1002/ijc.23418
Kannagi, R., Sakuma, K., Miyazaki, K., Lim, K.T., Yusa, A., Yin, J., Izawa, M.: Altered expression of glycan genes in cancers induced by epigenetic silencing and tumor hypoxia: clues in the ongoing search for new tumor markers. Cancer Sci 101, 586–593 (2010). https://doi.org/10.1111/j.1349-7006.2009.01455.x
Peixoto, A., Fernandes, E., Gaiteiro, C., Lima, L., Azevedo, R., Soares, J., Cotton, S., Parreira, B., Neves, M., Amaro, T., Tavares, A., Teixeira, F., Palmeira, C., Rangel, M., Silva, A.M.N., Reis, C.A., Santos, L.L., Oliveira, M.J., Ferreira, J.A.: Hypoxia enhances the malignant nature of bladder cancer cells and concomitantly antagonizes protein O-glycosylation extension. Oncotarget 7, 63138–63157 (2016). https://doi.org/10.18632/oncotarget.11257
Takamiya, R., Ohtsubo, K., Takamatsu, S., Taniguchi, N., Angata, T.: The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 23, 178–187 (2013). https://doi.org/10.1093/glycob/cws139
Julien, S., Lagadec, C., Krzewinski-Recchi, M.A., Courtand, G., Le Bourhis, X., Delannoy, P.: Stable expression of sialyl-Tn antigen in T47-D cells induces a decrease of cell adhesion and an increase of cell migration. Breast Cancer Res. Treat 90, 77–84 (2005). https://doi.org/10.1007/s10549-004-3137-3
Ogawa, T., Hirohashi, Y., Murai, A., Nishidate, T., Okita, K., Wang, L., Ikehara, Y., Satoyoshi, T., Usui, A., Kubo, T., Nakastugawa, M., Kanaseki, T., Tsukahara, T., Kutomi, G., Furuhata, T., Hirata, K., Sato, N., Mizuguchi, T., Takemasa, I., Torigoe, T.: ST6GALNAC1 plays important roles in enhancing cancer stem phenotypes of colorectal cancer via the akt pathway. Oncotarget 8, 112550–112564 (2017). https://doi.org/10.18632/oncotarget.22545
Ozaki, H., Matsuzaki, H., Ando, H., Kaji, H., Nakanishi, H., Ikehara, Y., Narimatsu, H.: Enhancement of metastatic ability by ectopic expression of ST6GalNAcI on a gastric cancer cell line in a mouse model. Clin. Exp. Metastasis 29, 229–238 (2012). https://doi.org/10.1007/s10585-011-9445-1
Albuquerque, A.P.B., Balmaña, M., Mereiter, S., Pinto, F., Reis, C.A., Beltrão, E.I.C.: Hypoxia and serum deprivation induces glycan alterations in triple negative breast cancer cells. Biol. Chem 399, 661–672 (2018). https://doi.org/10.1515/hsz-2018-0121
Wang, W.Y., Cao, Y.X., Zhou, X., Wei, B., Zhan, L., Sun, S.Y.: Stimulative role of ST6GALNAC1 in proliferation, migration and invasion of ovarian cancer stem cells via the akt signaling pathway. Cancer Cell. Int 19, 86 (2019). https://doi.org/10.1186/s12935-019-0780-7
Julien, S., Adriaenssens, E., Ottenberg, K., Furlan, A., Courtand, G., Vercoutter-Edouart, A.S., Hanisch, F.G., Delannoy, P., Le Bourhis, X.: ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 16, 54–64 (2006). https://doi.org/10.1093/glycob/cwj033
Maignien, C., Santulli, P., Chouzenoux, S., Gonzalez-Foruria, I., Marcellin, L., Doridot, L., Jeljeli, M., Grange, P., Reis, F.M., Chapron, C., Batteux, F.: Reduced α-2,6 sialylation regulates cell migration in endometriosis. Hum. Reprod 34, 479–490 (2019). https://doi.org/10.1093/humrep/dey391
Yu, X., Wu, Q., Wang, L., Zhao, Y., Zhang, Q., Meng, Q., Pawan, Wang, S.: Silencing of ST6GalNAc I suppresses the proliferation, migration and invasion of hepatocarcinoma cells through PI3K/AKT/NF-κB pathway. Tumour Biol 37, 12213–12221 (2016). https://doi.org/10.1007/s13277-016-5086-y
Peixoto, A., Freitas, R., Ferreira, D., Relvas-santos, M.: Metabolomics, transcriptomics and functional glycomics reveals bladder cancer cells plasticity and enhanced aggressiveness facing hypoxia and glucose deprivation. bioRxiv. 225084000 (2021). https://doi.org/10.1101/2021.02.14.431133
Santos, S.N., Junqueira, M.S., Francisco, G., Vilanova, M., Magalhães, A., Baruffi, M.D., Chammas, R., Harris, A.L., Reis, C.A., Bernardes, E.S.: O-glycan sialylation alters galectin-3 subcellular localization and decreases chemotherapy sensitivity in gastric cancer. Oncotarget 7, 83570–83587 (2016). https://doi.org/10.18632/oncotarget.13192
Miles, D.W., Happerfield, L.C., Smith, P., Gillibrand, R., Bobrow, L.G., Gregory, W.M., Rubens, R.D.: Expression of sialyl-Tn predicts the effect of adjuvant chemotherapy in node-positive breast cancer. Br. J. Cancer 70, 1272–1275 (1994). https://doi.org/10.1038/bjc.1994.486
Park, J.J., Yi, J.Y., Jin, Y.B., Lee, Y.J., Lee, J.S., Lee, Y.S., Ko, Y.G., Lee, M.: Sialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cells. Biochem. Pharmacol 83, 849–857 (2012). https://doi.org/10.1016/j.bcp.2012.01.007
Shen, L., Yu, M., Xu, X., Gao, L., Ni, J., Luo, Z., Wu, S.: Knockdown of β3GnT8 reverses 5-fluorouracil resistance in human colorectal cancer cells via inhibition the biosynthesis of polylactosamine-type N-glycans. Int. J. Oncol 45, 2560–2568 (2014). https://doi.org/10.3892/ijo.2014.2672
Wagner, K.W., Punnoose, E.A., Januario, T., Lawrence, D.A., Pitti, R.M., Lancaster, K., Lee, D., Von Goetz, M., Yee, S.F., Totpal, K., Huw, L., Katta, V., Cavet, G., Hymowitz, S.G., Amler, L., Ashkenazi, A.: Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med 13, 1070–1077 (2007). https://doi.org/10.1038/nm1627
Khiaowichit, J., Talabnin, C., Dechsukhum, C., Silsirivanit, A., Talabnin, K.: Down-regulation of C1GALT1 enhances the progression of cholangiocarcinoma through activation of AKT/ERK signaling pathways. Life 12, 174 (2022). https://doi.org/10.3390/life12020174
Gao, Y., Foster, R., Yang, X., Feng, Y., Shen, J.K., Mankin, H.J., Hornicek, F.J., Amiji, M.M., Duan, Z.: Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget 6, 9313–9326 (2015). https://doi.org/10.18632/oncotarget.3220
Nath, S., Daneshvar, K., Roy, L.D., Grover, P., Kidiyoor, A., Mosley, L., Sahraei, M., Mukherjee, P.: MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis 2, e51–e59 (2013). https://doi.org/10.1038/oncsis.2013.16
O’Neill, A.J., Prencipe, M., Dowling, C., Fan, Y., Mulrane, L., Gallagher, W.M., O’Connor, D., O’Connor, R., Devery, A., Corcoran, C., Rani, S., O’Driscoll, L., Fitzpatrick, J.M., Watson, R.W.G.: Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol. Cancer 10, 126 (2011). https://doi.org/10.1186/1476-4598-10-126
Kramer, R., Weber, T.K., Arceci, R., Ramchurren, N., Kastrinakis, W.V., Steele, G., Summerhayes, I.C.: Inhibition of N-linked glycosylation of P-glycoprotein by tunicamycin results in a reduced multidrug resistance phenotype. Br. J. Cancer 71, 670–675 (1995). https://doi.org/10.1038/bjc.1995.133
Greer, D.A., lvey, S.: Distinct N-glycan glycosylation of p-glycoprotein isolated from the human uterine sarcoma cell line MES-SA/DX5. Mol. Cell. Biochem 1770, 1275–1282 (2012). https://doi.org/10.1016/j.bbagen.2007.07.005
Very, N., Lefebvre, T., Yazidi-Belkoura, E.: Drug resistance related to aberrant glycosylation in colorectal cancer. Oncotarget 9, 1380–1402 (2017). https://doi.org/10.18632/oncotarget.22377
Lin, M.C., Chien, P.H., Wu, H.Y., Chen, S.T., Juan, H.F., Lou, P.J., Huang, M.C.: C1GALT1 predicts poor prognosis and is a potential therapeutic target in head and neck cancer. Oncogene 37, 5780–5793 (2018). https://doi.org/10.1038/s41388-018-0375-0
Tajadura-Ortega, V., Gambardella, G., Skinner, A., Halim, A., Van Coillie, J., Schjoldager, K.T.G., Beatson, R., Graham, R., Achkova, D., Taylor-Papadimitriou, J., Ciccarelli, F.D., Burchell, J.M.: O-linked mucin-type glycosylation regulates the transcriptional programme downstream of EGFR. Glycobiology 31, 200–210 (2021). https://doi.org/10.1093/glycob/cwaa075
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was partly supported by MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411003 to TS) and JSPS KAKENHI (JP23241075 to T.S.), Japan.
Author information
Authors and Affiliations
Contributions
Daiki Yamamoto and Toshinori Sato conceived and planned the experiments, and wrote the main manuscript text. Takeo Kosaka, Hiroshi Hongo and Mototsugu Oya helped supervise the project and contributed analysis tools. Daiki Yamamoto carried out the experiment, and Natsumi Aoki contributed sample preparation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.25 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamamoto, D., Hongo, H., Kosaka, T. et al. The sialyl-Tn antigen synthase genes regulates migration–proliferation dichotomy in prostate cancer cells under hypoxia. Glycoconj J 40, 199–212 (2023). https://doi.org/10.1007/s10719-023-10104-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-023-10104-z