Abstract
Background
The prescription of antidepressant drugs during pregnancy has been steadily increasing for several decades. Meta-analyses (MAs), which increase the statistical power and precision of results, have gained interest for assessing the safety of antidepressant drugs during pregnancy.
Objective
We aimed to provide a meta-review of MAs assessing the benefits and risks of antidepressant drug use during pregnancy.
Methods
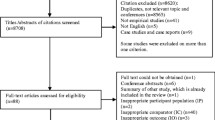
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a literature search on PubMed and Web of Science databases was conducted on 25 October, 2021, on MAs assessing the association between antidepressant drug use during pregnancy and health outcomes for the pregnant women, embryo, fetus, newborn, and developing child. Study selection and data extraction were carried out independently and in duplicate by two authors. The methodological quality of included studies was evaluated with the AMSTAR-2 tool. Overlap among MAs was assessed by calculating the corrected covered area. Data were presented in a narrative synthesis, using four levels of evidence.
Results
Fifty-one MAs were included, all but one assessing risks. These provided evidence for a significant increase in the risks for major congenital malformations (selective serotonin reuptake inhibitors, paroxetine, fluoxetine, no evidence for sertraline; eight MAs), congenital heart defects (paroxetine, fluoxetine, sertraline; 11 MAs), preterm birth (eight MAs), neonatal adaptation symptoms (eight MAs), and persistent pulmonary hypertension of the newborn (three MAs). There was limited evidence (only one MA for each outcome) for a significant increase in the risks for postpartum hemorrhage, and with a high risk of bias, for stillbirth, impaired motor development, and intellectual disability. There was inconclusive evidence, i.e., discrepant results, for an increase in the risks for spontaneous abortion, small for gestational age and low birthweight, respiratory distress, convulsions, feeding problems, and for a subsequent risk for autism with an early antidepressant drug exposure. Finally, MAs provided no evidence for an increase in the risks for gestational hypertension, preeclampsia, and for a subsequent risk for attention-deficit/hyperactivity disorder. Only one MA assessed benefits, providing limited evidence for preventing relapse in severe or recurrent depression. Effect sizes were small, except for neonatal symptoms (small to large). Results were based on MAs in which overall methodological quality was low (AMSTAR-2 score = 54.8% ± 12.9%, [19–81%]), with a high risk of bias, notably indication bias. The corrected covered area was 3.27%, which corresponds to a slight overlap.
Conclusions
This meta-review has implications for clinical practice and future research. First, these results suggest that antidepressant drugs should be used as a second-line treatment during pregnancy (after first-line psychotherapy, according to the guidelines). The risk of major congenital malformations could be prevented by observing guidelines that discourage the use of paroxetine and fluoxetine. Second, to decrease heterogeneity and bias, future MAs should adjust for maternal psychiatric disorders and antidepressant drug dosage, and perform analyses by timing of exposure.



Similar content being viewed by others
References
Molenaar NM, Bais B, Lambregtse-van den Berg MP, et al. The international prevalence of antidepressant use before, during, and after pregnancy: a systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J Affect Disord. 2020;264:82–9. https://doi.org/10.1016/j.jad.2019.12.014.
Andrade SE, Reichman ME, Mott K, et al. Use of selective serotonin reuptake inhibitors (SSRIs) in women delivering liveborn infants and other women of child-bearing age within the U.S. Food and Drug Administration’s Mini-Sentinel program. Arch Womens Ment Health. 2016;19(6):969–77. https://doi.org/10.1007/s00737-016-0637-1.
Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1-5. https://doi.org/10.1016/j.ajog.2007.01.033.
Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1-5. https://doi.org/10.1016/j.ajog.2007.07.036.
Charlton R, Jordan S, Pierini A, et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG An Int J Obstet Gynaecol. 2015;122(7):1010–20.
Zoega H, Kieler H, Nørgaard M, et al. Use of SSRI and SNRI antidepressants during pregnancy: a population-based study from Denmark, Iceland, Norway and Sweden. PLoS ONE. 2015;10(12):e0144474. https://doi.org/10.1371/journal.pone.0144474.
Ishikawa T, Obara T, Kikuchi S, et al. Antidepressant prescriptions for prenatal and postpartum women in Japan: a health administrative database study. J Affect Disord. 2020;264:295–303. https://doi.org/10.1016/j.jad.2020.01.016.
Hung C, Chan JKN, Wong CSM, Fung VSC, Lee KCK, Chang WC. Antidepressant utilization patterns and predictors of treatment continuation in pregnant women: a 16-year population-based cohort. Aust N Z J Psychiatry. 2022. https://doi.org/10.1177/00048674221109443.
Yin X, Sun N, Jiang N, et al. Prevalence and associated factors of antenatal depression: systematic reviews and meta-analyses. Clin Psychol Rev. 2021;83:101932. https://doi.org/10.1016/j.cpr.2020.101932.
Underwood L, Waldie K, Souza SD, Peterson ER, Morton S. A review of longitudinal studies on antenatal and postnatal depression. Arch Womens Ment Health. 2016;19(5):711–20. https://doi.org/10.1007/s00737-016-0629-1.
Schwalm M, Miotti H, Hellard C, Bounit L, Trehony J, Jouaville S. Treatment indications for antidepressants prescribed in primary care in France, 2006–2015. Value Health. 2016;19(7):A529. https://doi.org/10.1016/j.jval.2016.09.1062.
Molenaar NM, Kamperman AM, Boyce P, Bergink V. Guidelines on treatment of perinatal depression with antidepressants: an international review. Aust N Z J Psychiatry. 2018;52(4):320–7. https://doi.org/10.1177/0004867418762057.
Cabaillot A, Bourset A, Mulliez A, et al. Trajectories of antidepressant drugs during pregnancy: a cohort study from a community-based sample. Br J Clin Pharmacol. 2021;87(3):965–87. https://doi.org/10.1111/bcp.14449.
Fischer Fumeaux CJ, Morisod Harari M, Weisskopf E, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence: an update. Expert Opin Drug Saf. 2019;18(10):949–63. https://doi.org/10.1080/14740338.2019.1658740.
Bellantuono C, Vargas M, Mandarelli G, Nardi B, Martini MG. The safety of serotonin-noradrenaline reuptake inhibitors (SNRIs) in pregnancy and breastfeeding: a comprehensive review. Hum Psychopharmacol Clin Exp. 2015;30(3):143–51. https://doi.org/10.1002/hup.2473.
Gentile S. Tricyclic antidepressants in pregnancy and puerperium. Expert Opin Drug Saf. 2014;13(2):207–25. https://doi.org/10.1517/14740338.2014.869582.
Biffi A, Cantarutti A, Rea F, Locatelli A, Zanini R, Corrao G. Use of antidepressants during pregnancy and neonatal outcomes: an umbrella review of meta-analyses of observational studies. J Psychiatr Res. 2020;124:99–108. https://doi.org/10.1016/j.jpsychires.2020.02.023.
Ornoy A, Koren G. SSRIs and SNRIs (SRI) in pregnancy: effects on the course of pregnancy and the offspring: how far are we from having all the answers? Int J Mol Sci. 2019;20(10):2370. https://doi.org/10.3390/ijms20102370.
Biondi-Zoccai G. Umbrella reviews. In: Evidence synthesis with overviews of reviews and meta-epidemiologic studies. Cham: Springer International; 2016. https://doi.org/10.1007/978-3-319-25655-9.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Hennessy EA, Johnson BT, Keenan C. Best practice guidelines and essential methodological steps to conduct rigorous and systematic meta-reviews. Appl Psychol Health Well Being. 2019;11(3):353–81. https://doi.org/10.1111/aphw.12169.
Linde K. Systematic reviews and metaanalyses. In: Clinical research in complementary therapies. Churchill Livingstone; 2002, p. 187–97. https://doi.org/10.1016/B978-0-443-06367-1.50015-6.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. https://doi.org/10.1136/bmj.j4008.
Hennessy EA, Johnson BT. Examining overlap of included studies in meta-reviews: guidance for using the corrected covered area index. Res Synth Methods. 2020;11(1):134–45. https://doi.org/10.1002/jrsm.1390.
Pieper D, Antoine S-L, Mathes T, Neugebauer EAM, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75. https://doi.org/10.1016/j.jclinepi.2013.11.007.
Huis in het Veld JG, Verkaik R, Mistiaen P, van Meijel B, Francke AL. The effectiveness of interventions in supporting self-management of informal caregivers of people with dementia; a systematic meta review. BMC Geriatr. 2015;15(1):147. https://doi.org/10.1186/s12877-015-0145-6.
Vlenterie R, van Gelder MMHJ, Anderson HR, et al. Associations between maternal depression, antidepressant use during pregnancy, and adverse pregnancy outcomes. Obstet Gynecol. 2021;138(4):633–46. https://doi.org/10.1097/AOG.0000000000004538.
Bayrampour H, Kapoor A, Bunka M, Ryan D. The risk of relapse of depression during pregnancy after discontinuation of antidepressants. J Clin Psychiatry. 2020. https://doi.org/10.4088/JCP.19r13134.
Guan H-B, Wei Y, Wang L-L, Qiao C, Liu C-X. Prenatal selective serotonin reuptake inhibitor use and associated risk for gestational hypertension and preeclampsia: a meta-analysis of cohort studies. J Women’s Heal. 2018;27(6):791–800. https://doi.org/10.1089/jwh.2017.6642.
Jiang H, Xu L, Li Y, Deng M, Peng C, Ruan B. Antidepressant use during pregnancy and risk of postpartum hemorrhage: a systematic review and meta-analysis. J Psychiatr Res. 2016;83:160–7. https://doi.org/10.1016/j.jpsychires.2016.09.001.
ACOG. Early pregnancy loss. 2015. https://www.acog.org/womens-health/faqs/early-pregnancy-loss. Accessed 17 Feb 2023.
Xing D, Wu R, Chen L, Wang T. Maternal use of antidepressants during pregnancy and risks for adverse perinatal outcomes: a meta-analysis. J Psychosom Res. 2020;137:110231. https://doi.org/10.1016/j.jpsychores.2020.110231.
Nikfar S, Rahimi R, Hendoiee N, Abdollahi M. Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: a systematic review and updated meta-analysis. DARU J Pharm Sci. 2012;20(1):75. https://doi.org/10.1186/2008-2231-20-75.
Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication. JAMA Psychiat. 2013;70(4):436. https://doi.org/10.1001/jamapsychiatry.2013.684.
Einarson TR, Kennedy D, Einarson A. Do findings differ across research design? The case of antidepressant use in pregnancy and malformations. J Popul Ther Clin Pharmacol. 2012;19(2):e334–48.
Shen Z, Gao S, Li SX, et al. Sertraline use in the first trimester and risk of congenital anomalies: a systemic review and meta-analysis of cohort studies. Br J Clin Pharmacol. 2017;83(4):909–22. https://doi.org/10.1111/bcp.13161.
Turner E, Jones M, Vaz LR, Coleman T. Systematic review and meta-analysis to assess the safety of bupropion and varenicline in pregnancy. Nicotine Tob Res. 2019;21(8):1001–10. https://doi.org/10.1093/ntr/nty055.
De Vries C, Gadzhanova S, Sykes MJ, Ward M, Roughead E. A systematic review and meta-analysis considering the risk for congenital heart defects of antidepressant classes and individual antidepressants. Drug Saf. 2021;44(3):291–312. https://doi.org/10.1007/s40264-020-01027-x.
Selmer R, Haglund B, Furu K, et al. Individual-based versus aggregate meta-analysis in multi-database studies of pregnancy outcomes: the Nordic example of selective serotonin reuptake inhibitors and venlafaxine in pregnancy. Pharmacoepidemiol Drug Saf. 2016;25(10):1160–9. https://doi.org/10.1002/pds.4033.
Jordan S, Morris JK, Davies GI, et al. Selective serotonin reuptake inhibitor (SSRI) antidepressants in pregnancy and congenital anomalies: analysis of linked databases in Wales, Norway and Funen, Denmark. PLoS ONE. 2016;11(12):e0165122. https://doi.org/10.1371/journal.pone.0165122.
Wang S, Yang L, Wang L, Gao L, Xu B, Xiong Y. Selective serotonin reuptake inhibitors (SSRIs) and the risk of congenital heart defects: a meta-analysis of prospective cohort studies. J Am Heart Assoc. 2015;4(5):1–7. https://doi.org/10.1161/JAHA.114.001681.
Myles N, Newall H, Ward H, Large M. Systematic meta-analysis of individual selective serotonin reuptake inhibitor medications and congenital malformations. Aust N Z J Psychiatry. 2013;47(11):1002–12. https://doi.org/10.1177/0004867413492219.
Gao S-Y, Wu Q-J, Sun C, et al. Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: a systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med. 2018;16(1):205. https://doi.org/10.1186/s12916-018-1193-5.
Grigoriadis S, Graves L, Peer M, et al. Benzodiazepine use during pregnancy alone or in combination with an antidepressant and congenital malformations. J Clin Psychiatry. 2019. https://doi.org/10.4088/JCP.18r12412.
Zwink N, Jenetzky E. Maternal drug use and the risk of anorectal malformations: systematic review and meta-analysis. Orphanet J Rare Dis. 2018;13(1):75. https://doi.org/10.1186/s13023-018-0789-3.
Wurst KE, Poole C, Ephross SA, Olshan AF. First trimester paroxetine use and the prevalence of congenital, specifically cardiac, defects: a meta-analysis of epidemiological studies. Birth Defects Res Part A Clin Mol Teratol. 2010;88(3):159–70. https://doi.org/10.1002/bdra.20627.
Bérard A, Iessa N, Chaabane S, Muanda FT, Boukhris T, Zhao J-P. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81(4):589–604. https://doi.org/10.1111/bcp.12849.
Gao S-Y, Wu Q-J, Zhang T-N, et al. Fluoxetine and congenital malformations: a systematic review and meta-analysis of cohort studies. Br J Clin Pharmacol. 2017;83(10):2134–47. https://doi.org/10.1111/bcp.13321.
Riggin L, Frankel Z, Moretti M, Pupco A, Koren G. The fetal safety of fluoxetine: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2013;35(4):362–9. https://doi.org/10.1016/S1701-2163(15)30965-8.
Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. Antidepressant exposure during pregnancy and congenital malformations: is there an association? J Clin Psychiatry. 2013;74(04):e293-308. https://doi.org/10.4088/JCP.12r07966.
Moorthie S, Blencowe H, Darlison MW, et al. Estimating the birth prevalence and pregnancy outcomes of congenital malformations worldwide. J Community Genet. 2018;9(4):387–96. https://doi.org/10.1007/s12687-018-0384-2.
van Gelder MMHJ, van Rooij IALM, Miller RK, Zielhuis GA, de Jong-van den Berg LTW, Roeleveld N. Teratogenic mechanisms of medical drugs. Hum Reprod Update. 2010;16(4):378–94. https://doi.org/10.1093/humupd/dmp052.
Huecker MR, Smiley A, Saadabadi A. Bupropion. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 (PMID: 29262173). https://www.ncbi.nlm.nih.gov/books/NBK470212
Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48(2):455–63. https://doi.org/10.1093/ije/dyz009.
Huybrechts KF, Sanghani RS, Avorn J, Urato AC. Preterm birth and antidepressant medication use during pregnancy: a systematic review and meta-analysis. PLoS ONE. 2014;9(3):e92778. https://doi.org/10.1371/journal.pone.0092778.
Chang Q, Ma XY, Xu XR, Su H, Wu QJ, Zhao YH. Antidepressant use in depressed women during pregnancy and the risk of preterm birth: a systematic review and meta-analysis of 23 cohort studies. Front Pharmacol. 2020;11(May):1–12. https://doi.org/10.3389/fphar.2020.00659.
Huang H, Coleman S, Bridge JA, Yonkers K, Katon W. A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birth weight. Gen Hosp Psychiatry. 2014;36(1):13–8. https://doi.org/10.1016/j.genhosppsych.2013.08.002.
Eke AC, Saccone G, Berghella V. Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and risk of preterm birth: a systematic review and meta-analysis. BJOG An Int J Obstet Gynaecol. 2016;123(12):1900–7. https://doi.org/10.1111/1471-0528.14144.
McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period. Obstet Anesth Dig. 2015;35(3):126–7. https://doi.org/10.1097/01.aoa.0000469461.45993.de.
Kautzky A, Slamanig R, Unger A, Höflich A. Neonatal outcome and adaption after in utero exposure to antidepressants: a systematic review and meta-analysis. Acta Psychiatr Scand. 2022;145:6–28. https://doi.org/10.1111/acps.13367.
Zhao X, Liu Q, Cao S, et al. A meta-analysis of selective serotonin reuptake inhibitors (SSRIs) use during prenatal depression and risk of low birth weight and small for gestational age. J Affect Disord. 2018;241:563–70. https://doi.org/10.1016/j.jad.2018.08.061.
Black RE. Global prevalence of small for gestational age births. Nestle Nutr Inst Workshop Ser. 2015;81:1–7. https://doi.org/10.1159/000365790.
Say L, Donner A, Gülmezoglu AM, Taljaard M, Piaggio G. The prevalence of stillbirths: a systematic review. Reprod Health. 2006;3(1):1. https://doi.org/10.1186/1742-4755-3-1.
Corti S, Pileri P, Mazzocco MI, et al. Neonatal outcomes in maternal depression in relation to intrauterine drug exposure. Front Pediatr. 2019;7:1–8. https://doi.org/10.3389/fped.2019.00309.
Galbally M, Spigset O, Johnson AR, Kohan R, Lappas M, Lewis AJ. Neonatal adaptation following intrauterine antidepressant exposure: assessment, drug assay levels, and infant development outcomes. Pediatr Res. 2017;82(5):806–13. https://doi.org/10.1038/pr.2017.156.
Forsberg L, Navér L, Gustafsson LL, Wide K. Neonatal adaptation in infants prenatally exposed to antidepressants: clinical monitoring using neonatal abstinence score. PLoS ONE. 2014;9(11):e111327. https://doi.org/10.1371/journal.pone.0111327.
Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The effect of prenatal antidepressant exposure on neonatal adaptation. J Clin Psychiatry. 2013;74(04):e309–20. https://doi.org/10.4088/JCP.12r07967.
Wang J, Cosci F. Neonatal withdrawal syndrome following late in utero exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis of observational studies. Psychother Psychosom. 2021;90(5):299–307. https://doi.org/10.1159/000516031.
Leung MTY, Wong KH, Ho PWH, et al. Gestational exposure to antidepressants and risk of seizure in offspring: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;131:345–59. https://doi.org/10.1016/j.neubiorev.2021.09.040.
Martinho S, Adão R, Leite-Moreira AF, Brás-Silva C. Persistent pulmonary hypertension of the newborn: pathophysiological mechanisms and novel therapeutic approaches. Front Pediatr. 2020;8:342. https://doi.org/10.3389/fped.2020.00342.
Ng QX, Venkatanarayanan N, Ho CYX, Sim WS, Lim DY, Yeo W-S. Selective serotonin reuptake inhibitors and persistent pulmonary hypertension of the newborn: an updated meta-analysis. J Womens Health. 2019;28(3):331–8. https://doi.org/10.1089/jwh.2018.7319.
Masarwa R, Bar-Oz B, Gorelik E, Reif S, Perlman A, Matok I. Prenatal exposure to selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors and risk for persistent pulmonary hypertension of the newborn: a systematic review, meta-analysis, and network meta-analysis. Am J Obstet Gynecol. 2019;220(1):57.e1-57.e13. https://doi.org/10.1016/j.ajog.2018.08.030.
Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis. BMJ. 2014;348:f6932. https://doi.org/10.1136/bmj.f6932.
Lai MC, Lombardo MV, Baron-Cohen S. Autism Lancet. 2014;383(9920):896–910. https://doi.org/10.1016/S0140-6736(13)61539-1.
Xu G, Strathearn L, Liu B, et al. Prevalence and treatment patterns of autism spectrum disorder in the United States, 2016. JAMA Pediatr. 2019;173(2):153–9. https://doi.org/10.1001/jamapediatrics.2018.4208.
Rais TB, Rais A. Association between antidepressants use during pregnancy and autistic spectrum disorders: a meta-analysis. Innov Clin Neurosci. 2014;11(5–6):18–22.
Mezzacappa A, Lasica P-A, Gianfagna F, et al. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure. JAMA Pediatr. 2017;171(6):555. https://doi.org/10.1001/jamapediatrics.2017.0124.
Zhou X-H, Li Y-J, Ou J-J, Li Y-M. Association between maternal antidepressant use during pregnancy and autism spectrum disorder: an updated meta-analysis. Mol Autism. 2018;9(1):21. https://doi.org/10.1186/s13229-018-0207-7.
Halvorsen A, Hesel B, Østergaard SD, Danielsen AA. In utero exposure to selective serotonin reuptake inhibitors and development of mental disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2019;139(6):493–507. https://doi.org/10.1111/acps.13030.
Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med. 2018;16(1):6. https://doi.org/10.1186/s12916-017-0993-3.
Vega ML, Newport GC, Bozhdaraj D, Saltz SB, Nemeroff CB, Newport DJ. Implementation of advanced methods for reproductive pharmacovigilance in autism: a meta-analysis of the effects of prenatal antidepressant exposure. Am J Psychiatry. 2020;177(6):506–17. https://doi.org/10.1176/appi.ajp.2020.18070766.
Man KKC, Tong HHY, Wong LYL, Chan EW, Simonoff E, Wong ICK. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev. 2015;49(6):82–9. https://doi.org/10.1016/j.neubiorev.2014.11.020.
Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta-analysis. Reprod Toxicol. 2016;66:31–43. https://doi.org/10.1016/j.reprotox.2016.09.013.
Kobayashi T, Matsuyama T, Takeuchi M, Ito S. Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis. Reprod Toxicol. 2016;65:170–8. https://doi.org/10.1016/j.reprotox.2016.07.016.
Andalib S, Emamhadi MR, Yousefzadeh-Chabok S, et al. Maternal SSRI exposure increases the risk of autistic offspring: a meta-analysis and systematic review. Eur Psychiatry. 2017;45:161–6. https://doi.org/10.1016/j.eurpsy.2017.06.001.
Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies. Br J Clin Pharmacol. 2017;83(12):2798–806. https://doi.org/10.1111/bcp.13382.
Leshem R, Bar-Oz B, Diav-Citrin O, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) during pregnancy and the risk for autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) in the offspring: a true effect or a bias? A systematic review & meta-analysis. Curr Neuropharmacol. 2021;19(6):896–906. https://doi.org/10.2174/1570159X19666210303121059.
Csizmadi I, Collet J-P, Boivin J-F. Bias and confounding in pharmacoepidemiology. In: Pharmacoepidemiology. Chichester, UK: John Wiley & Sons, Ltd; p. 791–809. https://doi.org/10.1002/9780470059876.ch47.
Frisell T. Invited commentary: sibling-comparison designs, are they worth the effort? Am J Epidemiol. 2021;190(5):738–41. https://doi.org/10.1093/aje/kwaa183.
Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5(2):175–86. https://doi.org/10.1016/S2215-0366(17)30167-0.
Jiang H-Y, Peng C-T, Zhang X, Ruan B. Antidepressant use during pregnancy and the risk of attention-deficit/hyperactivity disorder in the children: a meta-analysis of cohort studies. BJOG An Int J Obstet Gynaecol. 2018;125(9):1077–84. https://doi.org/10.1111/1471-0528.15059.
Man KKC, Chan EW, Ip P, et al. Prenatal antidepressant exposure and the risk of attention-deficit hyperactivity disorder in children: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;86:1–11. https://doi.org/10.1016/j.neubiorev.2017.12.007.
Grove K, Lewis AJ, Galbally M. Prenatal antidepressant exposure and child motor development: a meta-analysis. Pediatrics. 2018;142(1):e20180356. https://doi.org/10.1542/peds.2018-0356.
Nisell H, Larsson G, Wager J. The relation between life stress and hypertensive complications during pregnancy. Acta Obstet Gynecol Scand. 1989;68(5):423–7. https://doi.org/10.3109/00016348909021014.
Kurki T. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95(4):487–90. https://doi.org/10.1016/S0029-7844(99)00602-X.
Price SM, Caughey AB. The impact of prenatal care on pregnancy outcomes in women with depression. J Matern Neonatal Med. 2022;35(20):3948–54. https://doi.org/10.1080/14767058.2020.1844655.
De Ocampo MPG, Araneta MRG, Macera CA, Alcaraz JE, Moore TR, Chambers CD. Risk of gestational hypertension and preeclampsia in women who discontinued or continued antidepressant medication use during pregnancy. Arch Womens Ment Health. 2016;19(6):1051–61. https://doi.org/10.1007/s00737-016-0655-z.
Bernard N, Forest J-C, Tarabulsy GM, Bujold E, Bouvier D, Giguère Y. Use of antidepressants and anxiolytics in early pregnancy and the risk of preeclampsia and gestational hypertension: a prospective study. BMC Pregnancy Childbirth. 2019;19(1):146. https://doi.org/10.1186/s12884-019-2285-8.
Andrade C, Sandarsh S, Chethan KB, Nagesh KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding. J Clin Psychiatry. 2010;71(12):1565–75. https://doi.org/10.4088/JCP.09r05786blu.
Arck PC, Rücke M, Rose M, et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed Online. 2008;17(1):101–13. https://doi.org/10.1016/S1472-6483(10)60300-8.
Marinescu IP, Foarfă MC, Pîrlog MC, Turculeanu A. Prenatal depression and stress: risk factors for placental pathology and spontaneous abortion. Rom J Morphol Embryol. 2014;55:1155–60.
Gentile S. Early pregnancy exposure to selective serotonin reuptake inhibitors, risks of major structural malformations, and hypothesized teratogenic mechanisms. Expert Opin Drug Metab Toxicol. 2015;11(10):1585–97. https://doi.org/10.1517/17425255.2015.1063614.
Heinonen E, Blennow M, Blomdahl-Wetterholm M, et al. Sertraline concentrations in pregnant women are steady and the drug transfer to their infants is low. Eur J Clin Pharmacol. 2021;77(9):1323–31. https://doi.org/10.1007/s00228-021-03122-z.
Chaabane S, Berard A. Epidemiology of major congenital malformations with specific focus on teratogens. Curr Drug Saf. 2013;8(2):128–40. https://doi.org/10.2174/15748863112079990011.
Swendsen J. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20(2):173–89. https://doi.org/10.1016/S0272-7358(99)00026-4.
Persson M, Cnattingius S, Villamor E, et al. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ. 2017;357:j2563. https://doi.org/10.1136/bmj.j2563.
Sharafi SE, Garmaroudi G, Ghafouri M, et al. Prevalence of anxiety and depression in patients with overweight and obesity. Obes Med. 2020;17:100169. https://doi.org/10.1016/j.obmed.2019.100169.
Le Gloan L, Legendre A, Iserin L, Ladouceur M. Pathophysiology and natural history of atrial septal defect. J Thorac Dis. 2018;10(S24):S2854–63. https://doi.org/10.21037/jtd.2018.02.80.
Daud A, Bergman J, Kerstjens-Frederikse W, Groen H, Wilffert B. The risk of congenital heart anomalies following prenatal exposure to serotonin reuptake inhibitors: is pharmacogenetics the key? Int J Mol Sci. 2016;17(8):1333. https://doi.org/10.3390/ijms17081333.
Benatar S, Cross-Barnet C, Johnston E, Hill I. Prenatal depression: assessment and outcomes among Medicaid participants. J Behav Health Serv Res. 2020;47(3):409–23. https://doi.org/10.1007/s11414-020-09689-2.
Fekadu Dadi A, Miller ER, Woodman RJ, Azale T, Mwanri L. Effect of antenatal depression on adverse birth outcomes in Gondar town, Ethiopia: a community-based cohort study. PLoS ONE. 2020;15(6):e0234728. https://doi.org/10.1371/journal.pone.0234728.
Mitchell J, Goodman J. Comparative effects of antidepressant medications and untreated major depression on pregnancy outcomes: a systematic review. Arch Womens Ment Health. 2018;21(5):505–16. https://doi.org/10.1007/s00737-018-0844-z.
Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early Hum Dev. 2009;85(1):65–70. https://doi.org/10.1016/j.earlhumdev.2008.07.002.
Goldstein DJ, Corbin LA, Sundell KL. Effects of first-trimester fluoxetine exposure on the newborn. Obstet Gynecol. 1997;89(5):713–8. https://doi.org/10.1016/S0029-7844(97)00070-7.
Kulin NA, Pastuszak A, Sage SR, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors; a prospective controlled multicenter study. JAMA. 1998;279(8):609–10. https://doi.org/10.1001/jama.279.8.609.
Einarson A, Bonari L, Voyer-Lavigne S, et al. A multicentre prospective controlled study to determine the safety of trazodone and nefazodone use during pregnancy. Can J Psychiatry. 2003;48(2):106–10. https://doi.org/10.1177/070674370304800207.
Sivojelezova A, Shuhaiber S, Sarkissian L, Einarson A, Koren G. Citalopram use in pregnancy: prospective comparative evaluation of pregnancy and fetal outcome. Am J Obstet Gynecol. 2005;193(6):2004–9. https://doi.org/10.1016/j.ajog.2005.05.012.
Chun-Fai-Chan B, Koren G, Fayez I, et al. Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. Am J Obstet Gynecol. 2005;192(3):932–6. https://doi.org/10.1016/j.ajog.2004.09.027.
Colvin L, Slack-Smith L, Stanley FJ, Bower C. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Res A Clin Mol Teratol. 2011;91(3):142–52.
Klieger-Grossmann C, Weitzner B, Panchaud A, et al. Pregnancy outcomes following use of escitalopram: a prospective comparative cohort study. J Clin Pharmacol. 2012;52(5):766–70. https://doi.org/10.1177/0091270011405524.
Kjaersgaard MIS, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion: a population-based study. PLoS ONE. 2013;8(8):e72095. https://doi.org/10.1371/journal.pone.0072095.
Stephansson O, Kieler H, Haglund B, et al. During pregnancy and risk of stillbirth description of sample. JAMA. 2013;309(1):48–54.
Wachman EM, Schiff DM, Silverstein M. Neonatal abstinence syndrome. JAMA. 2018;319(13):1362. https://doi.org/10.1001/jama.2018.2640.
Klinger G, Frankenthal D, Merlob P, et al. Long-term outcome following selective serotonin reuptake inhibitor induced neonatal abstinence syndrome. J Perinatol. 2011;31(9):615–20. https://doi.org/10.1038/jp.2010.211.
Alwan S, Bandoli G, Chambers C. Maternal use of selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. Clin Pharmacol Ther. 2016;100(1):34–41. https://doi.org/10.1002/cpt.376.
Daly E, D. Tricklebank M, Wichers R. Neurodevelopmental roles and the serotonin hypothesis of autism spectrum disorder. In: The serotonin system. Academic Press, 2019; p. 23–44. https://doi.org/10.1016/B978-0-12-813323-1.00002-5.
Kinast K, Peeters D, Kolk SM, Schubert D, Homberg JR. Genetic and pharmacological manipulations of the serotonergic system in early life: neurodevelopmental underpinnings of autism-related behavior. Front Cell Neurosci. 2013;7:72. https://doi.org/10.3389/fncel.2013.00072.
Caparros-Gonzalez RA, de la Torre-Luque A, Romero-Gonzalez B, Quesada-Soto JM, Alderdice F, Peralta-Ramírez MI. Stress during pregnancy and the development of diseases in the offspring: a systematic-review and meta-analysis. Midwifery. 2021;97(Jan):102939. https://doi.org/10.1016/j.midw.2021.102939.
Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562–75. https://doi.org/10.1038/s41380-018-0070-0.
Mayer JS, Bernhard A, Fann N, et al. Cognitive mechanisms underlying depressive disorders in ADHD: a systematic review. Neurosci Biobehav Rev. 2021;121:307–45. https://doi.org/10.1016/j.neubiorev.2020.12.018.
Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience. 2017;342:154–66. https://doi.org/10.1016/j.neuroscience.2015.09.001.
Molenaar NM, Brouwer ME, Kamperman AM, et al. Recurrence of depression in the perinatal period: clinical features and associated vulnerability markers in an observational cohort. PLoS ONE. 2019;14(2):e0212964. https://doi.org/10.1371/journal.pone.0212964.
Netsi E, Pearson RM, Murray L, Cooper P, Craske MG, Stein A. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiat. 2018;75(3):247. https://doi.org/10.1001/jamapsychiatry.2017.4363.
Slomian J, Honvo G, Emonts P, Reginster J-Y, Bruyère O. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health (Lond). 2019;15:174550651984404. https://doi.org/10.1177/1745506519844044.
Grigoriadis S, Graves L, Peer M, et al. Pregnancy and delivery outcomes following benzodiazepine exposure: a systematic review and meta-analysis. Can J Psychiatry. 2020;65(12):821–34. https://doi.org/10.1177/0706743720904860.
Källén B, Nilsson E, Olausson PO. Antidepressant use during pregnancy: comparison of data obtained from a prescription register and from antenatal care records. Eur J Clin Pharmacol. 2011;67(8):839–45. https://doi.org/10.1007/s00228-011-1021-8.
Habecker E, Freeman MP. Awareness and management of obstetrical complications of depression. Curr Psychiatr. 2015;14(12):39–44.
Matthias K, Rissling O, Pieper D, et al. The methodological quality of systematic reviews on the treatment of adult major depression needs improvement according to AMSTAR 2: A cross-sectional study. Heliyon. 2020;6(9):e04776. https://doi.org/10.1016/j.heliyon.2020.e04776.
Kieviet N, van Keulen V, van de Ven PM, Dolman KM, Deckers M, Honig A. Serotonin and poor neonatal adaptation after antidepressant exposure in utero. Acta Neuropsychiatr. 2017;29(1):43–53. https://doi.org/10.1017/neu.2016.30.
ACOG. ACOG practice bulletin no 92: use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001–20. https://doi.org/10.1097/AOG.0b013e31816fd910.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Conflicts of Interest/Competing Interests
Pierre Desaunay, Léa-Gabrielle Eude, Michel Dreyfus, Cénéric Alexandre, Sophie Fedrizzi, Joachim Alexandre, Faruk Uguz, and Fabian Guénolé have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate.
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
PD and LGE carried out the study selection and data extraction. All authors contributed equally to interpretation of the results, and revising the manuscript. All authors read and approved the final manuscript, and agreed to be accountable for the work. All authors meet the four ICMJE criteria for authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Desaunay, P., Eude, LG., Dreyfus, M. et al. Benefits and Risks of Antidepressant Drugs During Pregnancy: A Systematic Review of Meta-analyses. Pediatr Drugs 25, 247–265 (2023). https://doi.org/10.1007/s40272-023-00561-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00561-2