Abstract
Excessive exploitation, negligence, non-degradable nature, and physical and chemical properties of plastic waste have resulted in a massive pollution load into the environment. Consequently, plastic entres the food chain and can cause serious health issues in aquatic animals and humans. The present review summarizes currently reported techniques and approaches for the removal of plastic waste. Many techniques, such as adsorption, coagulation, photocatalysis, and microbial degradation, and approaches like reduction, reuse and recycling are potentially in trend and differ from each other in their efficiency and interaction mechanism. Moreover, substantial advantages and challenges associated with these techniques and approaches are highlighted to develop an understanding of the selection of possible ways for a sustainable future. Nevertheless, in addition to the reduction of plastic waste from the ecosystem, many alternative opportunities have also been explored to cash plastic waste. These fields include the synthesis of adsorbents for the removal of pollutants from aqueous and gaseous stream, their utility in clothing, waste to energy and fuel and in construction (road making). Substantial evidence can be observed in the reduction of plastic pollution from various ecosystems. In addition, it is important to develop an understanding of factors that need to be emphasized while considering alternative approaches and opportunities to cash plastic waste (like adsorbent, clothing, waste to energy and fuel). The thrust of this review is to provide readers with a comprehensive overview of the development status of techniques and approaches to overcome the global issue of plastic pollution and the outlook on the exploitation of this waste as resources.
Similar content being viewed by others
Introduction
Nowadays, it is fair to say plastics can be regarded as a sweet poison for both humans and the environment. Undoubtedly, plastics are important in day-to-day life because of their longevity (mechanical properties, thermal properties, stability, and durability), resistance to decomposition, and low cost, which support their widespread applicability [1,2,3]. The pioneering work done by Belgian-American chemist Leo Baekeland in 1907 was remarkable, leading to the mass production of the first real synthetic plastic known as Bakelite [4, 5]. Countless new plastics and their groups with desirable properties have been realized and synthesized. Plastic materials can be used as an alternative in place of materials, glass, wood, and metals, for construction, decoration, packaging, coatings, drawing into filaments, and weaving. Later, after their commercialization in 1964, plastics occupied their place in every home, office, factory, and vehicle, and became an intrinsic part of human life [6, 7].
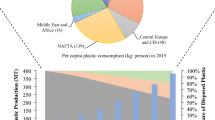
The plastic production rate continues to escalate, and global production dramatically reached 368 million metric tons in 2019 from 50 million metric tons in 1976. However, a short downturn can be observed in annual production in 2020 (367 million metric tons), which was predominantly the result of the COVID-19 pandemic. Among the number of plastics cumulatively produced, approximately 30% of plastics are still in use. Whereas approximately 55% of the total plastics were discarded, around 8% were incinerated, and only about 6% were recycled [8]. Before 1980, incineration and recycling methods were least prioritized or can say negligible.
After being used in specific applications for a period of time, plastics are considered as waste and ultimately discarded. With increasing population and development, the amount of discarded plastic increases at an alarming rate, and around 381 million tons of plastic polymers have been released [8]. Explosive population, industrial revolution, mismanagement approach, and physiochemical properties have led to an increase in discarded plastic waste [9, 10]. Consequently, they enter our nature and have a negative impact on the environment and human health with which they come in contact [11, 12]. As a result, their advantageous properties have now become a headache for researchers worldwide. Therefore, it is imperative to reduce the plastic waste load from the environmental ecosystem. The main thrust of the present article is to scratch the relevant information about the generation of plastic waste, disposal, along with the impact on nature, and mitigation. The initial desk research was conducted from various published articles and online sources such as Scopus Index databases and Science Citation Index Expanded (SCI-E), Environmental Performance Index (EPI), and United Nations Development Programme (UNEP) to gather relevant information. Article reflects a brief sketch of various techniques (such as adsorption, coagulation, photocatalysis, and microbial degradation) and approaches (such as reduction, reuse and recycling) that have withdrawn researchers’ attention to eliminate plastic pollution. Furthermore, their efficiency, interaction mechanism, advantages, and challenges are also explored. This article also highlights the alternative approaches and opportunities to cash plastic waste like adsorbent, clothing, waste to energy, and fuel along with prospects.
Synthesis and chemical nature
Depending on the starting materials and reaction involved in the synthesis different types of plastic polymers have been developed including: polyethylene terephthalate, high-density polyethylene, polyvinyl chloride, low-density polyethylene, polypropylene, polystyrene, and others [13,14,15].
Commercial plastic products take into account intricate mixtures of chemicals and probe additives with the aim of improving the basic mechanical, physical, or chemical properties of the plastic product as per the requirement. Table 1 summarizes the types of plastic, their application and properties. Additionally, additives shelter the polymer from the degrading effects of heat, light, heat, or bacteria and provide special characteristics such as improved surface appearance, reduced friction, and flame retardancy [16].
Structure of polymers
The plastic structure usually depends on the linkage of carbon atoms, either may be homopolymer or copolymer. The structures that include the linkage of carbon-to-carbon atoms are considered as homogeneous polymers, such as PP, polybutylene, PS, and polymethyl pentene [28]. However, if the linkage of the carbon atoms is interrupted by oxygen or nitrogen atoms, the structure known as a heterogeneous polymer, such as polyesters, nylons, and polycarbonates, is formed. In such structures, different elements can be attached to the carbon-to-carbon backbone, such as PVC containing attached chlorine atoms, and Teflon containing attached fluorine atoms [23, 29]. The plastics can also be altered by the insertion of some additives. In Table 2, some additives (used to alter the properties of commercial plastic polymers) are discussed along with their application.
Consequences of using plastic
Generation of various pollutants and issues associated with plastic waste
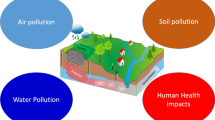
The disposed plastic waste materials react with their surroundings and release several degradation products, such as additives and polymers (of which they are made), throughout their lifetime [37]. Released chemicals (additives), plastic type, and their size determine the extent of the impact on the environment and human health [38, 39]. Plastic waste itself is solid waste that is classified as microplastic and microplastic. Waste with a diameter size > 20 mm is considered macroplastics, whereas, waste with an upper size limit of 5 mm in diameter is considered as microplastic. These microplastics are further characterized as primary microplastics (manufactured to have a size less than 5 mm) and secondary microplastics (resultant of degradation of macroplastics, found in textiles, medicines, and personal care products like facial and body scrubs) [40, 41]. Plastic after its utility is over is sent to landfills or burning in open or closed environments and is vulnerable to human health, air, water, and soil. When plastics are disposed over territorial areas, they turn out to be a prime source for solid waste problems, and as they ingress into water bodies (ultimately travel to an ocean), they become a serious threat to aquatic animals, as shown in Fig. 1 [42]. This illustration shows the pathway of traveling plastic waste from a residential or industrial area to their surroundings due to improper disposal. These plastic wastes (such as single-use plastics and others) float or move in surrounds either by air, water streams, and other activities (likes by animals), which finally end up in the ocean. As a result, this seriously impacts humans and other aquatic animals.
The fumes generated after burning plastic waste contain hazardous compounds like halogenated additives, PCBs, PVCs, fumes, dioxins, carbon monoxide (CO), arsenic, mercury, carbon dioxide (CO2), methane (CH4), and volatile organic compounds (VOCs) [37, 43, 44]. Some compounds like CO2 and CH4 are liable to stimulate the greenhouse effect, which increase the Earth’s temperature and contribute to climate change [45, 46].
Impact on environment and human
The human inhales byproduct of plastic waste burning in the atmosphere may include VOCs, dioxins, and CO, which may cause health-related issues such as cancer, endometriosis, birth defects, and child developmental disorders. These compounds can trigger neurological damage [47], reproductive issue [48], immune-related issue [49], asthma [50], and endocrine disruption [51, 52], even at low concentrations. Table 3 indicates various additives and other chemicals released from plastic components and their associated health issues.
Substances, such as Arsenic (As), Pb, Cadmium (Cd), and Mercury (Hg) are considered heavy metals, are sufficient to degrade water quality, and can reduce soil fertility at low concentrations [65, 68]. Moreover, we can frequently observe various plastic products (macroplastics or microplastics) in water bodies (rivers, streams, ponds, and oceans). These plastics are generated in the terrestrial region, end up in the oceans and degrade the health of the ecosystem [69,70,71]. Much evidence has been published that reflects aquatic species such as turtles, seabirds, fish, and marine mammals are suffering from entanglement, often associated with large animals when comparing to ingestion (visible in smaller animals) [72, 73]. In addition, micro-sized plastics also lead to inflammation, traverse cellular barriers, and even cross highly selective membranes such as the blood–brain barrier or the placenta. Within the cell, they sometimes trigger changes in gene expression and biochemical reactions. The signs of chemicals released during degradation may cause acute and chronic effects on living beings and the ecosystem as well. Some of the causes have been investigated as disruptions in the hormone system of vertebrates and invertebrates alike [74,75,76,77].
The presence of plastics or other larger bodies can be easily detected and separated from water bodies. Presently, microplastic (< 10–6) and nanoplastics (< 10–9) are considered as emerging contaminants [78], whose micro/nano size limits their detection in water bodies and no standardized method has been recognized for their effective identification and quantification. Various studies have documented the presence of microplastics in the guts of fishes and the stomachs of many terrestrial animals [76, 79,80,81].
Moreover, we have limited data documented on chemicals associated with plastics. However, our above paragraph concluded that every human and animal is now the host for some concentration of chemicals associated with plastics and can also be found in air, water, and soil. Some of them may be: Polychlorinated biphenyls (PCBs), organochlorine pesticides (DDTs, HCHs, and HCB), polycyclic aromatic hydrocarbons (PAHs), brominated flame retardants (PBDE and HBCD), phthalates, UV stabilizers, and antioxidants, which have been widely detected in microplastic samples from water [82,83,84].
Impact of plastic waste on environmental performance index (EPI) of nation
Plastic pollution directly or indirectly affects human health and the environment. On the other hand, plastic pollution also affects the environmental performance ranking of any nation. Environmental ranking indicates the quality of the environment and the efforts made to enhance the status of the environment of the nation. The considered indicators in EPI collectively depict the status of 10 years of the environmental pathway for any nation. Moreover, it indicates the best countries addressing the environmental challenges that every nation faces. Yale University and Columbia University, in collaboration with the World Economic Forum, developed a biennial index that was first published in 2002. It offers a scorecard, ranking 180 countries, using 32 performance indicators considering 11 issues that highlight the leaders and laggards in environmental performance. Furthermore, it also provides practical guidance for countries that aspire to move toward a sustainable future [85, 86]. Figure 2 indicates all 32 performance indicators considering 11 issues that highlight the environmental performance of any nation. This EPI reflects the status of the nation in dealing with environmental pollution to overcome. Notably, the indicators used to evaluate the EPI are the sectors that can be altered by plastic waste. Hence, with the perception of the nation, it is imperative to reduce the plastic load from the environment.
Different technologies and approaches for the removal of plastic
Since the last decade, efforts have been made to eliminate plastic pollution either by reducing it at their source or by removing it after their generation. Various technologies, such as adsorption [87, 88], photocatalytic degradation [89, 90], coagulation [91], and microbial decomposition [92, 93] have been introduced and investigated to reduce plastic loads [94]. Along with this, approaches such as landfill, incineration, and 3R (reduce, reuse, and recycle) have also been coped up [95]. In the context, Fig. 3 shows the many sources responsible for plastic waste in nature and their serious impact. Figure 3 also illustrates various techniques and approaches to reduce plastic waste.
Adsorption
Adsorption, among the oldest techniques, has been successfully explored for the removal of organic and inorganic pollutants from water and wastewater. This is the surface phenomenon where pollutants from the water or gaseous phase are transferred to the surface of solid materials, called adsorbents. In this phenomenon, the adsorbent plays a vital role in the adsorption of pollutants [96, 97]. Recently, the adsorption technique has gained a lot of attention for the removal of microplastics from water.
Yuan et al. used three-dimensional reduced graphene oxide for the removal of microplastics from water. Its adsorption performance was highly dependent on pH, ion concentration, adsorption time, initial concentration, and temperature, and its maximum adsorption capacity was calculated as 617.28 mg/g at 26 °C. The higher adsorption efficiency was the result of strong π–π force with the help of electrostatic attraction and physical retention [92].
Wang et al. studied a natural and biodegradable sponge material (through chemical cross-linking using plant protein) with high mechanical properties for the adsorption of microplastic. The prepared material shows a removal efficiency of up to 81.2% in the pH range of 6–9 with an initial microplastic concentration of 1 mg/L. They aim to develop good mechanical strength materials with a compressive strength of 176 kPa and Young’s modulus of 60.1 kPa. The mechanical properties of the sponge remained up to 90% even after 100 compression cycles at a compressive strain of 70%, which shows commendable fatigue-resistance close to that of commercial polyurethane sponges. The abundant active side chains on amino acid residues provided the protein sponge with a good capacity to adsorb microplastics. The adsorption kinetic study suggested that hydrophobic interactions and the intra-particle diffusion drove the adsorption process. The sponge possessed a highly interconnected porous structure (83%), thus showing fast adsorption ability to microplastics with 38% adsorbed onto the sponge within 10 s. The entrapped water can be released from the sponge simply by squeezing for cyclic use, and the fast adsorption ability was still maintained after 20 cycles [98].
Ye et al. demonstrated the facile and scalable fabrication of two types of bubble-propelled iron oxides-MnO2 core–shell micromotors (Fe3O4-MnO2 and Fe2O3-MnO2) for the removal of suspended microplastics. They used micro/nanoscale manipulation (MNM) in various environmental remediations, such as microplastic removal, via the synergy of catalytic degradation, surface adsorption, and adsorptive bubble separation mechanisms. The adsorptive bubbles was found to efficiently separate more than 10% of the suspended microplastics from the polluted water in 2 h [99].
Ye et al. demonstrated magnetic carbon nanotubes (M−CNTs) for the removal of microplastic from an aqueous phase. In this approach, M−CNTs were effectively adsorbed on microplastic materials such as PE, PET, and polyamide (PA). The formed MPs/M− CNTs composites were then separated by magnetic force from aqueous solutions. The results show that the prepared materials were more efficient for PE followed by PET and PA (maximum adsorption capacities were 1650, 1400, and 1100 M−CNTs mg/g, respectively). Furthermore, the recycling of adsorbed M−CNTs via thermal treatment (600 °C) makes them more efficient with the same magnetic properties. In the study, they used M-CNTs four times and were still able to remove ~ 80% of total MPs in the testing solution [100].
Kim et al. investigated the application of granular activated carbon (GAC) for the removal of plastic (microplastic). GAC was found to be effective for PE and PP, including 20–50 µm microplastics. These compounds are lighter than water, which is rarely removed in primary and biological treatments. About MP removal efficiency, similar results were obtained for the DWTP (drinking water treatment plant) equipped with the GAC filtration system (88% cumulative removal) [101]. Moreover, adsorbents prepared from plastic waste without the addition of any environmental-related problematic raw materials or products have gained much attention. Carbon nanostructure derived from plastic waste embedded with Co3Fe7/CoFe2O4 magnetic nanoparticles can be effectively used for the removal of pollutants such as methyl orange and methylene blue from the aqueous phase [102].
The adsorption technique was found to be effective for emerging pollutants in water and air stream. The removal of microplastics can be easily adsorbed and separated from wastewater through various micro, meso, and macroporous nanomaterials. These techniques are limited to plastic size >5 µm. The removal of microplastic from oceans is an untouchable field with this technique. Moreover, the regeneration of adsorbents is also considered a limitation, which allows researchers to look forward to a more effective technique.
Photocatalysis
Photocatalysis is an exciting approach for handling plastic waste, especially micro/nano plastics. This technique is a light-mediated redox process, in which appropriate light energy excites nanostructured semiconductors that lead to the creation of exciton pairs, which react with surrounding water/moisture to produce highly reactive species like superoxides and hydroxyl radicals that can effectively oxidize organic species, including polymers [103]. Various photocatalysts, such as zinc oxide (ZnO) and titanium oxide (TiO2), have been used to study the degradation of PVC, PET, PS, and PE.
Tofa et al. demonstrated the successful degradation of LDPE film using ZnO nanorod photocatalysts. The photo-mediated oxidation process was prevalent over the cracks and spots on the LDPE film after 175 h of exposure. As a result, under optimum light energy, photocatalyst excitement leads to the formation of hydroxyl radicals, which have a high oxidation capacity for degrading organic pollutants [104].
Shang et al. [105] investigated the photocatalytic degradation of PS plastic over copper phthalocyanine (CuPc) sensitized TiO2 photocatalyst (TiO2/CuPc) under fluorescent light irradiation. In their result, UV−VIS spectra show that TiO2/CuPc extends its photoresponse range to visible light, contrasting to only UV light absorption of pure TiO2. For PS photodegradation, they observed a higher PS weight loss rate, lower PS average molecular weight, fewer volatile organic compounds, and more CO2 in the PS−(TiO2/CuPc) system than in the PS−TiO2 system. Their study suggests the potential application of dye-sensitized TiO2 catalysts in the thorough photodegradation of PS plastic under fluorescent light. The photodegradation over TiO2/CuPc composite is more competent and efficient than that over pure TiO2. Zhao et al. [106] evaluated the same photocatalyst for the degradation of PE. Their results show that PE-TiO2/CuPc has a higher photocatalytic degradation rate than PE-TiO2. Furthermore, PS-TiO2/CuPc has slower degradation than PE-TiO2/CuPc due to the different molecular structures [105].
Fa et al. studied the photocatalytic degradation of polyethylene-oxidized polyethylene wax-TiO2 (PE-OPW-TiO2). In their study to improve the degradation efficiency, they used OPW to enhance the interaction between PE and modified TiO2 particles [107].
Wang et al. [108] prepared magnetic Au@mag@TiO2 micromotors using TiO2 particles coated with 10 nm nickel and 30 nm gold. The prepared photocatalytic micromotor can move efficiently in water and peroxide when irradiated with UV light, depending on a photocatalytic chemical reaction. In the study, they used polystyrene (PS) passive particles as a model system for microplastics, and in a mixture of both, the Au@Ni@TiO2 micromotors collectted polystyrene particles very efficiently through phoretic interactions in 1.67% hydrogen peroxide solution with 315 mW UV light. In the study, they used the second method, shoveling (pushing method), for the removal of microplastics from a 0.2% hydrogen peroxide solution [108].
Iqra Nabi investigated the photocatalytic degradation of PS and PE microplastic under UV light irradiation over TiO2 nanoparticle films. The study shows the complete mineralization (98.40%) of photocatalyst in 12 h made with Triton X-100. High photodegradation rates were observed after 36 h for PE, and CO2 was found to be the main end product [109].
Abdusalam Uheida et al. demonstrated that glass fiber substrates could be efficiently used to trap low-density microplastics such as PP from water. They used ZnO nanorods (ZnO NRs) immobilized onto glass fiber substrates for visible light to degrade the microplastic [110].
Degradation of types of plastic with UV–VIS light excited heterogeneous ZnO and TiO2 photocatalysts lead to the formation of such intermediate products that could be further exploited as raw materials for the chemical industry for the production of new petrochemical products or plastics or in organic synthesis. Our limited knowledge and few publications about the photocatalytic degradation of plastics, especially nano plastic is considered as an emerging issue of great concern.
Coagulation
Coagulation is the chemical method for the removal of solid particles from water by introducing small and highly charged molecules into the water to manipulate the electrostatic charges of the suspended particles in water [111]. Limited data are presented on the application of this technique for the removal of microplastics.
Ma et al. [112] investigated the micro-sized PE behavior during removal from water with the help of Fe-based salt. They observed that the traditional coagulation process can have higher removal for smaller PE particles. They evaluated the effect of anionic polyacrylamide on the solution, which played a significant role in increasing the removal efficiency, especially anionic polyacrylamide at high dosages (with efficiency up to 90.9%). This is because a dense floc was formed and had high adsorption ability due to the positively charged Fe-based flocs under neutral conditions. However, for ultrafiltration, microplastic were completely rejected, and slight membrane fouling was caused owing to their large particle size. After the coagulation, membrane flux was decreased; however, less severe membrane fouling was induced by flocs. This is the result of the heterogeneous nature of the cake layer caused by PE, even at high dosages of Fe-based salts. They concluded that the behavior exhibited during coagulation and ultrafiltration could potentially be applied in drinking water treatment [112]. Baiwen Ma [112] evaluated the removal efficiency of Al- and Fe-based salts for the removal of PE from water which is the main constituent of microplastics. They observed that Al-based salts dominated over Fe-based salts in PE removal efficiency. The smaller the PE particle size was, the higher the removal efficiency. However, a low removal efficiency was observed, even with a high Al-based salt dosage of 15 mmol/L (below 40%). Additionally, the removal efficiency was barely influenced by water conditions, such as ionic strength, and turbidity level [112].
Zhou et al. studied the removal of PS and PE microplastics using PAC and ferric chloride coagulation, where PAC showed higher removal efficiency than ferric chloride. Their work concluded that the removal efficiency of microplastics under alkaline conditions was higher than that under acidic conditions. Chlorine ion Cl− had little effect on the removal efficiency of microplastics, while SO42− and CO32− had inhibitory and promoting effects, respectively [113].
Horn [114] investigated the removal of the model microplastic spheres and microfibers in their study using alum as the coagulant. They used the coagulant, alum at concentrations between 5 mg/L and 10 mg/L Al to produce water with turbidity less than 1.0 NTU from solutions containing 5 mg/L microspheres with an initial turbidity of 16 NTU. The occurrence of surfactant at 20 mg/L in solution does not negatively impact the performance of coagulations of microspheres at low alum. However, they may have a small detrimental effect at high particle loading and high alum doses. They predicted that the stability of polyethylene microfibers in water was strongly influenced by surfactants. The smallest polyethylene fibers could be dispersed in solution by a surfactant and effectively removed via coagulation [115].
Darabi et al. [116] investigated the removal efficiency of micro-and nano plastics (180–125 μm) during drinking water treatment, particularly coagulation/flocculation combined with sedimentation (CFS) and granular filtration under ordinary working conditions at water treatment plants (WTPs). It also studied the interactions between biofilms and microplastics and the consequential impact on treatment efficiency. Generally, CFS was not sufficient to remove micro and nano plastics. The sedimentation rate of clean plastics was lower than 2.0% for all different sizes of plastic particles with the coagulant Al2(SO4)3. Even with the addition of coagulant aid (PolyDADMAC), the highest removal was only 13.6% for 45–53 μm particles. In contrast, granular filtration was much more effective at filtering out micro and nano plastics, from 86.9% to nearly complete removal (99.9% for particles larger than 100 μm). However, there existed a critical size (10–20 μm) where a significantly lower removal (86.9%) was observed. Biofilms were easily formed on microplastics. In addition, biofilm formation significantly increased the removal efficiency of CFS treatment from <2.0% to 16.5%. This work provides new knowledge to better understand the fate and transport of emerging micro-and nano plastic pollutants during drinking water treatment, which is of increasing concern due to the potential human exposure to micro-and nano plastics in drinking water [117].
Microorganism
Nowadays, researchers and scientists have shifted their effort to develop strains that could potentially help eliminate plastic waste. Various microorganisms, such as bacteria, and fungus, have been reported to potentially degrade plastic waste, especially microplastics [118, 119]. In microbial degradation, plastics decompose and end up as biomass, methane, carbon dioxide, water, and other inorganic compounds [120]. In a recent report, a fungus was examined that breaks down the plastic sponge and assimilates it like any other food. Such studies are in progress evaluating their efficiency on PET and polyurethane.
Paço et al. examined the naturally occurring fungus Zalerion Maritimum, that potentially biodegrade polyethylene plastics [121]. Other lab-engineered versions bacteria like E. coli are under investigation to transform terephthalic acid, a molecule derived from PET, into the culinary flavouring vanillin, via a series of chemical reactions. However, this study is still at a very early stage, and need to do more to find ways to make the process more efficient and economically viable. Bacterial strain Pseudomonas sp. TDA1 is under investigation and was originally found in a local rubbish dump to break down polyurethane using the enzyme. This bacterium multiplies its biomass, consumes around half the plastic, and releases the rest as carbon dioxide [122]. This method is the slowest among all removal methods and takes ample days for degradation, which depends on the characteristics of plastic waste, and its physical and chemical nature. In addition, environmental factors such as temperature, sunlight, ultraviolet rays, and atmospheric humidity, can influence their degradation rate [92, 93].
Tournier [123] prepared an improved PET hydrolase that depolymerizes 90& PET into monomers with a productivity of 16.7 g of terephthalate per liter per hour (200 g/kg of PET suspension, with an enzyme concentration of 3 mg/g of PET) in 10 h. The optimized PET hydrolases reported thus far, including an enzyme from the bacterium Ideonella sakaiensis strain 201-F6 (even assisted by a secondary enzyme) and this variant, attracted recent interest [123].
Enzyme or microbial conversion of PET to its constituent building blocks is interesting science and needs to be explored. In this context, French company Carbios produces engineered enzymes that could break down PET. Carbios teamed up with L'Oreal and Nestle to produce enzymatically recycled the world's first food-grade PET plastic. The study confirms that these enzymes do not cause contamination if the packaging is still dirty. Moreover, they are very specific and energy-efficient (they do not use much energy). However, this technology is limited to two polyesters, signifying around 75 million tonnes of annual production, compared to a global plastics production of around 350 million tonnes.
Kim et al. [101] investigated worms that can consume polystyrene to a strain of bacteria that lives in the larvae’s gut. They placed polystyrene as the only carbon source with 50 superworms in a chamber and observed that the worms had consumed around 70% of the plastic in the first 21 days. Furthermore, a strain of Pseudomonas aeruginosa bacteria was isolated from the gut of the worms and showed that is can grow directly on the surface of polystyrene and break it down. Finally, an enzyme called serine hydrolase was identified from the bacteria as a responsible candidate for most of the biodegradation [124]. Currently, studies are more concentrated on exploring enzymes and microorganisms that use plastics as their source of energy, as a result, they can degrade plastic waste completely.
Landfill and incineration
A few years before, the landfill has been the preferred approach for plastic waste management in which capital includes low investment, easy construction, easy disposal, convenience, and approachability [125, 126]. Communities generally dispose of their waste at a particular place near residence or segregate in different dustbins that are finally dumped at particular sites (city outskirt). Decades to decades of practice have now resulted in release of the plastic waste into the ocean, in which landfill practices have played a key role in their travel [52, 70]. A study conducted estimates that before 2015, more than 8.3 billion metric tonnes of plastics were produced worldwide, of which only 9% was recycled and the rest ended up in landfills or the environment [8, 127]. In 2018 and 2019, more than 7.2 million tons of plastic were sent to landfills, and various valuable elements were also thrown away. The amount of plastic in landfills increases with the increase in plastic every year (as we produce around 300 million tonnes of plastic waste every year and that is equivalent to the weight of the entire human population) [128,129,130]. Consequently, some of the associated toxic chemicals leach out from the landfill site to the soil and groundwater, causing severe health issues. Therefore, due to environmental and health concerns, and the loss of valuable substances, they are now the least preferred management option [131, 132]. Many countries have imposed restrictions on landfills and put extra effort to recover value substances that reduce the amount of plastic waste at landfill sites. This approach adopted by European state members reduced the plastic waste sent to landfills, which hoard approximately 5.7 million tons of resources, by 44% between 2006 and 2018 [133, 134].
Developing and underdeveloped countries still prefer landfills because of low investment, ease of construction, and approachability. On the other hand, low literacy, lack of awareness of the environment, and plastic and casual approaches could be reasons [135,136,137,138]. It is estimated that an astounding 6500 tonnes of plastic waste are going to landfills per day. Some developed countries, such as the USA and Canada, export their plastic wastes to developing countries for processing due to low-wage workers and cheap processing charges [139]. The exported plastic waste is turned into a useful product, and the rest is either sent to a landfill or disposed to the ocean. As a result, the volume of plastic waste becomes a nuisance for everyone.
To reduce the volume of waste, researchers have tried to capitalize on the incineration approach. Incineration is always practiced as an alternative to landfills in which waste is thermally treated under a controlled combustion process (rapid oxidation) to reduce the volume of waste and to recover energy [140, 141]. However, incineration could be considered an the efficient approach to reduce plastic, but a disadvantage associated with it is noxious fumes, which are more dangerous. The fumes release VOCs, polychlorinated biphenyls (PCBs), polyvinyl chloride, dioxins, furans, CO, and CO2 from the incinerator depending on the type of waste [142,143,144,145,146]. Among some of the gages are considered greenhouse gages that lead to climate change [46, 147]. In addition, the incineration approach requires high installation investment, operation, and maintenance that restrict its application for plastic waste removal [148, 149]. These conditions insist on the 3R approach, i.e., Reduce, Reuse and Recycle.
Reduce, reuse and recycle (3R approach)
Alongside the application of the above-discussed techniques, other approaches (such as Reduction, Reuse, and Recycle) to lower plastic waste have importance. In this context, an individual’s behavior and consciousness play a key role in controlling the plastic load in the environment. For any country, awareness and education are the two pillars on which the 3R approach stands and are supported by other factors, like population restriction, attitude, knowledge, and practices. Reduction is always considered the best way to manage any waste [150, 151]. The key factor is reducing the purchasing amount or restricting only to buying what we need. This approach is not only limited to saving resources and energy but also reduces the number of pollutant emissions and subsequently improves the environment. Unfortunately, due to the limited availability of alternatives to plastic, the topmost priority approach shifted to the least priority. In the reference, Table 4 summarizes the comparison of various techniques and approaches, which highlights their advantages and disadvantages. However, various governmental and non-government efforts have been made to avoid the utilization of plastic products. In some European countries like Germany, in 2016, the step forward is to curb plastic bags or to apply a fee of €0.05 to €0.50 (about $0.06 to $0.60) on purchase. The agreement was made by the Ministry, the German Retail Federation, and participating companies to restrict the use of plastic bags. In 2016, Switzerland government and the Canadian government charged consumers some taxes to ban plastic bags, (around $0.04 per single-use plastic bag and $0.02 per reusable bag). As a result, the demand for plastic bags drastically decreased by 80%. Some commendable efforts were made by Asian countries like Indonesia, in 2017 to completely phase out plastic bags by 2018 [152].
Reuse is another approach that may reduce the demand for the new plastic product. However, many people prefer the recycling or refuse approach over reuse but the reuse approach saves money, and conserves energy and resources [153,154,155,156]. It is observed that the reuse approach has been maximumly capitalized in developing countries like India, Pakistan, and Bangladesh, where one can reuse sandwiches, plastic grocery bags for small trash bags, and re-use plastic silverware [157,158,159]. Reuse is an efficient approach for the reduction of plastic waste load but recycling is considered more effective methodology to restrict the utilization of virgin materials in the manufacturing of plastic products. Further, recycling could save 40–100 MJ/kg (depending on the polymer) energy and reduce the depletion of fossil fuels [160]. However, many countries have debated the preferences among reuse or recycle, but it is observed that recycling generally takes less energy than making plastic from raw materials. Reusing is preferred to using plastic waste, which consumes less energy and fewer resources. In recycling, the formation of different products depends on the type of chemical used, and this approach opens new pathways for road construction, toilet making and information on pavement blocks. Plastic waste materials are used for making Plastone, which is used as a binder aggregating solid that can be used in the construction of the structure of toilet blocks and construction of pavement blocks. It is interesting to mention that for making a single Plastone block, 300 plastic carry bags and 4 to 6 PET bottles are used [161]. Their high transverse strength results in providing long life which can be used for flooring especially outdoors, in raising compound walls and lining of the canal [162, 163].
Conversion of plastics into carbon nanostructures for energy and environmental application
Beyond the removal of plastic waste and restrictions on its generation, the conversion of plastic waste into useful resources like carbon-based material is a growing field. In this context, plastic wastes can be thermally treated to obtain carbon materials using methods such as anoxic pyrolysis, catalytic carbonization, pressure carbonization, flash Joule heating, microwave treatment, molten salt methods [164, 165], and hydrothermal methods [166, 167]. These treatments are often applied to achieve the desired products, such as carbon-based solids, which may include carbon nanotubes (CNT) [168], graphene [96, 169], hierarchically porous carbon (HPC) [117, 170], and carbon nanosheets [171, 172]. Plastic-derived carbon nanostructures can be used in energy storage devices such as supercapacitors and batteries, which promote sustainable green energy systems.
The materials with distinctive features like high surface area, high conductivity, and porosity can be used as hydrogen evolution reaction (HER) catalysts and oxygen evolution reaction (OER) catalysts in the water-splitting process.
In recent years, there has been growing interest in making adsorbents from waste, especially using waste plastics. Many methodologies have been documented to prepare the activated carbons and their potential application as adsorbents for the removal of dyes, heavy metals, and pharmaceuticals from wastewater. Generally, PET and polyurethane foam (PUF) waste has been exploited for the synthesis of activated carbon and graphene. The synthesized adsorbents have been investigated for the adsorptive removal of various emerging pollutants, such as methylene blue, acid blue 25, p-nitrophenol (PNP), Fe, cephalexin (CEX), and CO2, from wastewater and air [173, 174]. This effort helps in managing plastic waste and releases pressure by removing various organic and inorganic pollutants from wastewater. However, in this approach, making adsorbents from plastic waste at a large scale has many constraints. Combustion is one of the major causes of air pollution, generating pollutants such as alkanes, PAHs (including triphenylbenzenes), acids (e.g., terephthalic and 4-hydroxybenzoic), and phthalates [175, 176]. In addition, the capital cost is another factor restricting plastic incineration [156].
However, in the search for alternative fuels and energy, the higher calorific value of plastics than wood-based oil, which is comparable to conventional diesel, has drawn attention over their exploitation [177]. Many researchers exploit this opportunity to better utilize plastic waste. In this pyrolysis, the methodology is used to derive a useful product in absence of oxygen. Shafferina Dayana Anuar Sharuddin [178] applied this method (at 500 °C in presence of nitrogen gas) to investigate the potential production of liquid fuel based on their different compositions of plastic mainly derived from non-recycled plastics (NRPs) [178]. Many studies have shown the application of thermochemical pyrolysis technology for the conversion of municipal mixed plastic waste (MMPW) into high-quality hydrocarbon fuel. To enhance their efficiency and fuel productivity, a low-cost catalyst, i.e., CAT-1 of 10% (in weight), was used. These catalysts, however, were more efficient for the conversion of municipal virgin plastic than MMPW.
Future aspects and suggestions
The industries involved in manufacturing any plastic product either at any stage of manufacturing (raw material collection, processing, and packaging) should reserve 25% surface area of the plastic product to advertise against the use of plastic products, employing at least 25% surface area of the product (Fig. 4).
Even we can make alternations in clothing fashion. Here we suggest one more zip lock pocket in front of the jeans or pants to collect the wraps of chocolate, biscuits, chewing gums, etc. Daily collected plastic waste will be disposed of in the evening in proper waste collection dustbins. This approach avoids throwing waste plastic into our surroundings.
Implementation and consideration of plastic pollution as an indicator under the parameter of solid waste indicator in the environmental performance index will rank the countries based on their production and mitigation approaches. The best-ranked countries will be the path makers for lower-ranked countries. It is important to issue directions for the restricted utility of plastic at different shops which may include restaurants, confectionaries, hotels, vegetable shops, and especially unauthorized shops in developing countries. Customer and shop owner pay penalty schemes should be incorporated in the offence.
The implementation of a circular plastic economy could increase the reusability and recycling of plastic waste. It is observed that the linear plastic economy adopted sees 90% of products used once and then discarded. In a circular economy, plastic waste can be exploited as raw materials to develop and design various products such as pots, key rings, and decorative items. This concept helps to reduce the high volume of plastic and could be a source of income [96].
Unfortunately, an increase in pollution increases the product demand that ultimately directly or indirectly increases the utilization of plastic products and the high probability of plastic mismanagement. Hence, the population is the largest source of pollution; therefore, strategies should be developed and implemented to overcome such issues.
Traditional means of bleaching and screening are an effective approach for the removal of large plastic plastics. However, fabric nets of different sizes are applied at the source and exit in houses, industry, hospitals, and drainage to collect the entered plastic waste in the water stream.
In the chemical approach for plastic removal, different types of adsorbents such as pillared clays (PILCs), Porous Clay Hetersostructures (PCHs), MOFS, and activated carbon from different sources, could be potential candidates for microplastic removal.
Coupling different approaches, such as activated biological treatment followed by filtration and membrane filtration, may offer promising benefits.
Conclusions
The present review article provides an overview of the status of plastics and their waste, which are considered as one of the biggest threats to territorial life, aquatic life, and humans. Mishandling plastic waste releases toxic chemicals such as CO2, CH4, arsenic, and VOCs that may cause neurological damage, reproductive damage, immune damage, asthma, and endocrine disruption to humans even at low concentrations and contribute to climate change. Additionally, some by-products from plastic waste are considered hazardous to human health and can cause cancer, endometriosis, birth defects, and child developmental disorders. The plastic waste and its handling can affect the environmental performance ranking of any nation. Various technologies and approaches have been implemented to overcome plastic pollution. Adsorption technology, coagulation, photocatalysis, and microbial decomposition may remove plastics from the environment, although they are effective for microplastics. Understanding the interaction mechanism between plastic chemicals over adsorbents, coagulants, and photocatalyst. Nevertheless, landfill is always considered and prioritized for plastic waste management. Increasing volume of waste at landfills and adverse effects on soil, water, air, humans, territorial animals, and aquatic animals insist on incineration although it has a huge impact on the environment. Cause and effect pressurized to look after these approaches. Reduction, reuse, and recycling could be more effective in eliminating plastic waste, and coping with advanced technology offers enhanced efficiency. Reduction is considered the worth option but is an unavoidable approach, whereas the recycling approach is considered a better alternative in exploiting plastic waste in an alternate manner, such as adsorbents, fuels, energy, bricks, roads, and pavement, opening a new pathway to reduce their amount in nature as a waste. The implementation of a circular economy can play an effective role in reducing the volume of plastic waste which can be turned into many useful products. This concept can boost the economy of a nation and provide an alternative source of income. It is also important to couple technologies such as adsorption, coagulation, and photochemical degradation with the 3R approach to understand and develop better opportunities to save resources.
References
Brydson, J.A. 1999. Plastics materials. Amsterdam, the Netherlands: Elsevier.
Chen, G.G.Q. 2009. Plastics from bacteria: Natural functions and applications. Heidelberg, Germany: Springer Science.
Van den Tempel, M. 1961. Mechanical properties of plastic-disperse systems at very small deformations. Journal of Colloid Science 16 (3): 284–296.
Geyer, R. 2020. A brief history of plastics. In Mare Plasticum-The Plastic Sea, pp. 31–47. Berlin, Germany: Springer.
Gilbert, M. 2017. Plastics materials: Introduction and historical development. In Brydson’s plastics materials, pp. 1–18. Cham, Switzerland: Elsevier.
Rodrigues, M., Abrantes, N., Gonçalves, F., et al. 2019. Impacts of plastic products used in daily life on the environment and human health: What is known? Environmental Toxicology and Pharmacology 72: 103239.
Thompson, R.C., Swan, S.H., Moore, C.J., et al. 2009. Our plastic age. https://doi.org/10.1098/rstb.2009.0054.
Ritchie, H., and Roser, M. 2018. Plastic pollution. Oxford, UK: Our world in data.
Meides, N., Menzel, T., Poetzschner, B.R., et al. 2021. Reconstructing the environmental degradation of polystyrene by accelerated weathering. Environmental Science and Technology 55 (12) 7930–7938. https://doi.org/10.1021/acs.est.0c07718.
Singh, P., and Sharma, V. 2016. Integrated plastic waste management: environmental and improved health approaches. Procedia Environmental Sciences 35: 692–700.
Lau, W.W., Shiran, Y., Bailey, R.M., et al. 2020. Evaluating scenarios toward zero plastic pollution. Science 369 (6510): 1455–1461.
Rhodes, C.J. 2018. Plastic pollution and potential solutions. Science Progress 101 (3): 207–260.
Encinar, J., and González, J. 2008. Pyrolysis of synthetic polymers and plastic wastes. Kinetic study. Fuel Processing Technology 89 (7): 678–686.
Jung, M.R., Horgen, F.D., Orski, S.V., et al. 2018. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Marine Pollution Bulletin 127: 704–716.
Schwarz, A.E., Ligthart, T.N., Boukris, E., et al. 2019. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Marine Pollution Bulletin 143: 92–100.
Stipek, J., and Daoust, H. 2012. Additives for plastics. New York, NY, USA: Springer.
Kawai, F., Kawabata, T., and Oda, M. 2020. Current state and perspectives related to the polyethylene terephthalate hydrolases available for biorecycling. ACS Sustainable Chemistry and Engineering 8 (24): 8894–8908.
Adhikary, K.B., Pang, S., and Staiger, M.P. 2008. Dimensional stability and mechanical behaviour of wood–plastic composites based on recycled and virgin high-density polyethylene (HDPE). Composites, Part B: Engineering 39 (5): 807–815.
Kumar, S., Panda, A.K., and Singh, R.K. 2011. A review on tertiary recycling of high-density polyethylene to fuel. Resources, Conservation and Recycling 55 (11): 893–910.
Akovali, G. 2012. Plastic materials: polyvinyl chloride (PVC). In Toxicity of building materials, pp. 23–53. Sawston, UK: Woodhead Publishing.
Mercea, P.V., Losher, C., Petrasch, M., et al. 2018. Migration of stabilizers and plasticizer from recycled polyvinylchloride. Journal of Vinyl and Additive Technology 24: E112–E124.
Sogancioglu, M., Yel, E., and Ahmetli, G. 2017. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. Journal of Cleaner Production 165: 369–381.
Maddah, H.A. 2016. Polypropylene as a promising plastic: A review. American Journal of Polymer Science 6 (1): 1–11.
Ranjan, V.P., and Goel, S. 2021. Recyclability of polypropylene after exposure to four different environmental conditions. Resources, Conservation and Recycling 169: 105494.
Andrade, B.T.N.C., Bezerra, A.C., and Calado, C.R. 2019. Adding value to polystyrene waste by chemically transforming it into sulfonated polystyrene. Matéria (Rio de Janeiro) 24 (3). https://doi.org/10.1590/s1517-707620190003.0732.
Gao, J., Shen, Y., and Xu, H. 2016. Investigations for the mechanical, macro-, and microstructural analyses of dissimilar submerged friction stir welding of acrylonitrile butadiene styrene and polycarbonate sheets. Proceedings of the Institution of Mechanical Engineers, Part B 230 (7): 1213–1220.
Ţiplica, G.-S., Bucur, L., Bucur, G., et al. 2020. Other plastics. In Kanerva’s occupational dermatology, pp. 821–839. Berlin, Germany: Springer.
Lin, L., and Argon, A. 1994. Structure and plastic deformation of polyethylene. Journal of Materials Science 29 (2): 294–323.
Aggarwal, S. 1976. Structure and properties of block polymers and multiphase polymer systems: An overview of present status and future potential. Polymer 17 (11): 938–956.
Harris, R.M. 1999. Coloring technology for plastics. Norwich, NY, USA: William Andrew.
Heck, R.L., III. 1998. A review of commercially used chemical foaming agents for thermoplastic foams. Journal of Vinyl and Additive Technology 4 (2): 113–116.
Wensing, M., Uhde, E., and Salthammer, T. 2005. Plastics additives in the indoor environment—Flame retardants and plasticizers. Science of the Total Environment 339 (1–3): 19–40.
GmbH, C., and Richter, E. 2000. Lubricants for film manufacture. Plastics, Additives and Compounding 2 (11): 14–21.
Grob, M.C., and Minder, E. 1999. Permanent antistatic additives: New developments. Plastics, Additives and Compounding 1 (3): 20–26.
Jones, A. 2009. Choosing antimicrobial additives for plastics. Plastics, Additives and Compounding 11 (4): 26–28.
Papazoglou, E.S. 2004. Flame retardants for plastics. In Handbook of building materials for fire protection, pp. 41–488. New York, NY, USA: McGraw-Hill.
Verma, R., Vinoda, K., Papireddy, M., et al. 2016. Toxic pollutants from plastic waste-a review. Procedia Environmental Sciences 35: 701–708.
Alabi, O.A., Ologbonjaye, K.I., Awosolu, O., et al. 2019. Public and environmental health effects of plastic wastes disposal: A review. Journal of Toxicology and Risk Assessment 5 (021): 1–13.
Rustagi, N., Pradhan, S., and Singh, R. 2011. Public health impact of plastics: An overview. Indian Journal of Occupational and Environmental Medicine 15 (3): 100.
Laskar, N., and Kumar, U. 2019. Plastics and microplastics: A threat to environment. Environmental Technology and Innovation 14: 100352.
Lusher, A.L., Hernandez-Milian, G., O’Brien, J., et al. 2015. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environmental Pollution 199: 185–191.
Avio, C.G., Gorbi, S., and Regoli, F. 2017. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Marine Environmental Research 128: 2–11.
Cai, C., Yu, S., Liu, Y., et al. 2018. PBDE emission from E-wastes during the pyrolytic process: Emission factor, compositional profile, size distribution, and gas-particle partitioning. Environmental Pollution 235: 419–428.
Valavanidis, A., Iliopoulos, N., Gotsis, G., et al. 2008. Persistent free radicals, heavy metals and PAHs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. Journal of Hazardous Materials 156 (1–3): 277–284.
Kurdikar, D., Fournet, L., Slater, S.C., et al. 2000. Greenhouse gas profile of a plastic material derived from a genetically modified plant. Journal of Industrial Ecology 4 (3): 107–122.
Shen, M., Huang, W., Chen, M., et al. 2020. (Micro) plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. Journal of Cleaner Production 254: 120138.
Anwar, Z., Anjum, F., and Ghayas, S. 2021. Polycarbonate plastics and neurological disorders: From exposure to preventive interventions environmental contaminants and neurological disorders. In Environmental Contaminants and Neurological Disorders, pp. 147–183. Cham, Switzerland: Springer.
Amereh, F., Babaei, M., Eslami, A., et al. 2020. The emerging risk of exposure to nano (micro) plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environmental Pollution 261: 114158.
Navarro, G. P. P. 2020. Micro-plastics, nano-plastics and it’s components on the impact of human health. Doctoral dissertation, Lietuvos sveikatos mokslų universitetas.
Slovak, A. 1981. Occupational asthma caused by a plastics blowing agent, azodicarbonamide. Thorax 36 (12): 906–909.
Halden, R.U. 2010. Plastics and health risks. Annual Review of Public Health 31: 179–194.
Rajmohan, K.V.S., Ramya, C., Viswanathan, M.R., et al. 2019. Plastic pollutants: Effective waste management for pollution control and abatement. Current Opinion in Environmental Science and Health 12: 72–84.
Zhang, H., Xu, Y., Jia, L., et al. 2021. Smog chamber study on the ozone formation potential of acetaldehyde. Advances in Atmospheric Sciences 38 (7): 1238–1251.
Folkins, I., and Chatfield, R. 2000. Impact of acetone on ozone production and OH in the upper troposphere at high NOX. Journal of Geophysical Research: Atmospheres 105 (D9): 11585–11599.
Sankar, M., Nowicka, E., Carter, E., et al. 2014. The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol. Nature Communications 5 (1): 1–6.
Zubair, M., and Adrees, A. 2019. Dioxins and furans: Emerging contaminants of air. In Air pollution-monitoring, quantification and removal of gases and particles, pp. 111–125. London, UK: Intech Open.
Amthor, J. 1991. Respiration in a future, higher-CO2 world. Plant, Cell & Environment 14 (1): 13–20.
Yoshida, N., and Mizutani, Y. 1986. Preparation of carbon dioxide for oxygen-18 determination of water by use of a plastic syringe. Analytical Chemistry 58 (6): 1273–1275.
Nkwachukwu, O.I., Chima, C.H., Ikenna, A.O., et al. 2013. Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries. International Journal of Industrial Chemistry 4 (1): 1–13.
Wagoner, J.K. 1983. Toxicity of vinyl chloride and poly (vinyl chloride): A critical review. Environmental Health Perspectives 52: 61–66.
Lomonaco, T., Manco, E., Corti, A., et al. 2020. Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: A neglected source of environmental pollution. Journal of Hazardous Materials 394: 122596.
Royer, S.-J., Ferrón, S., Wilson, S.T., et al. 2018. Production of methane and ethylene from plastic in the environment. PLoS ONE 13 (8): e0200574.
Ross, S., and Evans, D. 2003. The environmental effect of reusing and recycling a plastic-based packaging system. Journal of Cleaner Production 11 (5): 561–571.
Abelsohn, A., Sanborn, M.D., Jessiman, B.J., et al. 2002. Identifying and managing adverse environmental health effects: 6. Carbon Monoxide Poisoning. Canadian Medical Association Journal 166 (13): 1685–1690.
Boyle, D., Catarino, A.I., Clark, N.J., et al. 2020. Polyvinyl chloride (PVC) plastic fragments release Pb additives that are bioavailable in zebrafish. Environmental Pollution 263: 114422.
Casas, J.S., and Sordo, J. 2011. Lead: chemistry, analytical aspects, environmental impact and health effects. Amsterdam, the Netherlands: Elsevier.
Rosen, J.F. 1995. Adverse health effects of lead at low exposure levels: trends in the management of childhood lead poisoning. Toxicology 97 (1–3): 11–17.
Saini, V., and Pandey, P. 2017. Natural husks as potential adsorbents for uptake of heavy metals. Millersville PA, USA: Materials Research Foundations.
Jambeck, J.R., Geyer, R., Wilcox, C., et al. 2015. Plastic waste inputs from land into the ocean. Science 347 (6223): 768–771.
Kershaw, P., Katsuhiko, S., Lee, S., et al. 2011. Plastic debris in the ocean. United Nations Environment Programme.
Wabnitz, C., and Nichols, W.J. 2010. Plastic pollution: An ocean emergency. Marine Turtle Newsletter 129: 1.
Hoarau, L., Ainley, L., Jean, C., et al. 2014. Ingestion and defecation of marine debris by loggerhead sea turtles, Caretta caretta, from by-catches in the South-West Indian Ocean. Marine Pollution Bulletin 84 (1–2): 90–96.
Lavers, J.L., Hutton, I., and Bond, A.L. 2019. Clinical pathology of plastic ingestion in marine birds and relationships with blood chemistry. Environmental Science and Technology 53 (15): 9224–9231.
Beaumont, N.J., Aanesen, M., Austen, M.C., et al. 2019. Global ecological, social and economic impacts of marine plastic. Marine Pollution Bulletin 142: 189–195.
Dris, R., Imhof, H., Sanchez, W., et al. 2015. Beyond the ocean: Contamination of freshwater ecosystems with (micro-) plastic particles. Environmental Chemistry 12 (5): 539–550.
Gunaalan, K., Fabbri, E., and Capolupo, M. 2020. The hidden threat of plastic leachates: A critical review on their impacts on aquatic organisms. Water Research 184: 116170.
Peng, L., Fu, D., Qi, H., et al. 2020. Micro-and nano-plastics in marine environment: Source, distribution and threats—A review. Science of the total environment 698: 134254.
Chen, Z., Fang, J., Wei, W., Ngo, H.H., Guo, W., Ni, B.J. 2022. Emerging adsorbents for micro/nanoplastics removal from contaminated water: Advances and perspectives. Journal of Cleaner Production. 19: 133676.
Chae, Y., and An, Y.-J. 2017. Effects of micro-and nanoplastics on aquatic ecosystems: Current research trends and perspectives. Marine Pollution Bulletin 124 (2): 624–632.
Franzellitti, S., Canesi, L., Auguste, M., et al. 2019. Microplastic exposure and effects in aquatic organisms: A physiological perspective. Environmental Toxicology and Pharmacology 68: 37–51.
Gao, G., Zhao, X., Jin, P., et al. 2021. Current understanding and challenges for aquatic primary producers in a world with rising micro-and nano-plastic levels. Journal of Hazardous Materials 406: 124685.
Vethaak, A.D., and Leslie, H.A. 2016. Plastic debris is a human health issue. Cham, Switzerland: ACS Publications.
Waring, R.H., Harris, R., and Mitchell, S. 2018. Plastic contamination of the food chain: A threat to human health? Maturitas 115: 64–68.
Wright, S.L., and Kelly, F.J. 2017. Plastic and human health: a micro issue? Environmental Science and Technology 51 (12): 6634–6647.
Environmental Performance Index. 2018. Environmental performance index. New Haven, CT, USA: Yale University and Columbia University.
Ma, Z., Ryberg, M.W., Wang, P., et al. 2020. China’s import of waste PET bottles benefited global plastic circularity and environmental performance. ACS Sustainable Chemistry and Engineering 8 (45): 16861–16868.
Tiwari, E., Singh, N., Khandelwal, N., et al. 2020. Application of Zn/Al layered double hydroxides for the removal of nano-scale plastic debris from aqueous systems. Journal of Hazardous Materials 397: 122769.
Wei, S., and Kamali, A.R. 2022. Trifunctional mesoporous magnetic adsorbent-photocatalyst nanocomposite for efficient removal of potassium ethyl xanthate from mining wastewater. Journal of Water Process Engineering 1 (49): 103067.
Nabi, I., Ahmad, F., and Zhang, L. 2021. Application of titanium dioxide for the photocatalytic degradation of macro-and micro-plastics: A review. Journal of Environmental Chemical Engineering 9: 105964.
Sarwan, B., Acharya, A.D., Kaur, S., et al. 2020. Visible light photocatalytic deterioration of polystyrene plastic using supported BiOCl nanoflower and nanodisk. European Polymer Journal 134: 109793.
Xu, Q., Huang, Q.-S., Luo, T.-Y., et al. 2021. Coagulation removal and photocatalytic degradation of microplastics in urban waters. Chemical Engineering Journal 416: 129123.
Yuan, F., Yue, L., Zhao, H., et al. 2020. Study on the adsorption of polystyrene microplastics by three-dimensional reduced graphene oxide. Water Science and Technology 81 (10): 2163–2175.
Yuan, J., Ma, J., Sun, Y., et al. 2020. Microbial degradation and other environmental aspects of microplastics/plastics. Science of the Total Environment 715: 136968.
Kundu, A., Shetti, N.P., Basu, S., et al. 2021. Identification and removal of micro-and nano-plastics: Efficient and cost-effective methods. Chemical Engineering Journal 421: 129816.
Hopewell, J., Dvorak, R., and Kosior, E. 2009. Plastics recycling: Challenges and opportunities. Philosophical Transactions of the Royal Society B 364 (1526): 2115–2126.
Pandey, P., and Saini, V.K. 2018. Pillared interlayered clays for pollution remediation. In Green adsorbents for pollutant removal, pp. 353–376. Cham, Switzerland: Springer.
Pandey, P., and Saini, V.K. 2019. Pillared interlayered clays: sustainable materials for pollution abatement. Environmental Chemistry Letters 17 (2): 721–727.
Wang, Z., Sun, C., Li, F., et al. 2021. Fatigue resistance, re-usable and biodegradable sponge materials from plant protein with rapid water adsorption capacity for microplastics removal. Chemical Engineering Journal 415: 129006.
Ye, H., Wang, Y., Liu, X., et al. 2021. Magnetically steerable iron oxides-manganese dioxide core–shell micromotors for organic and microplastic removals. Journal of Colloid and Interface Science 588: 510–521.
Tang, Y., Zhang, S., Su, Y., et al. 2021. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chemical Engineering Journal 406: 126804.
Kim, K.T., and Park, S. 2021. Enhancing microplastics removal from wastewater using electro-coagulation and granule-activated carbon with thermal regeneration. Processes 9 (4): 617.
Shi, H., Reza, M.O., Nikrityuk, P.A. 2021. The impact of swirling on the dynamics of a spouted bed. Powder Technology. 380: 143–51.
Ameta, R., Solanki, M.S., Benjamin, S., et al. 2018. Photocatalysis advanced oxidation processes for waste water treatment, pp. 135–175. Amsterdam, the Netherlands: Elsevier.
Tofa, T.S., Kunjali, K.L., Paul, S., et al. 2019. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environmental Chemistry Letters 17 (3): 1341–1346.
Shang, J., Chai, M., and Zhu, Y. 2003. Photocatalytic degradation of polystyrene plastic under fluorescent light. Environmental Science and Technology 37 (19): 4494–4499.
Zhao, X., Li, Z., Chen, Y., Shi, L., Zhu, Y. 2008. Enhancement of photocatalytic degradation of polyethylene plastic with CuPc modified TiO2 photocatalyst under solar light irradiation. Applied Surface Science. 254 (6): 1825–1829.
Fa, W., Yang, C., Gong, C., et al. 2010. Enhanced photodegradation efficiency of polyethylene-TiO2 nanocomposite film with oxidized polyethylene wax. Journal of Applied Polymer Science 118 (1): 378–384.
Wang, L., Kaeppler, A., Fischer, D., et al. 2019. Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter. ACS Applied Materials and Interfaces 11 (36): 32937–32944.
Nabi, I., Li, K., Cheng, H., et al. 2020. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. Iscience 23 (7): 101326.
Uheida, A., Mejía, H.G., Abdel-Rehim, M., et al. 2021. Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. Journal of hazardous materials 406: 124299.
O’Melia, C.R. 1978. Coagulation in wastewater treatment. In The scientific basis of flocculation, pp. 219–268. Dordrecht, Germany: Springer.
Ma, B., Xue, W., Ding, Y., et al. 2019. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. Journal of Environmental Sciences 78: 267–275.
Zhou, G., Wang, Q., Li, J., et al. 2021. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Science of the Total Environment 752: 141837.
Horn, D., Miller, M., Anderson, S., et al. 2019. Microplastics are ubiquitous on California beaches and enter the coastal food web through consumption by Pacific mole crabs. Mar Pollut Bull. 139: 231–237. https://doi.org/10.1016/j.marpolbul.2018.12.039.
Skaf, D.W., Punzi, V.L., Rolle, J.T., et al. 2020. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chemical Engineering Journal 386: 123807.
Darabi, M., Majeed, H., Diehl, A., Norton, J., Zhang, Y. 2021. A review of microplastics in aquatic sediments: occurrence, fate, transport, and ecological impact. Current Pollution Reports. 7 (1): 40–53.
Zhang, Y., Diehl, A., Lewandowski, A., et al. 2020. Removal efficiency of micro-and nanoplastics (180 nm–125 μm) during drinking water treatment. Science of the Total Environment 720: 137383.
Kyrikou, I., and Briassoulis, D. 2007. Biodegradation of agricultural plastic films: A critical review. Journal of Polymers and the Environment 15 (2): 125–150.
Shen, M., Zeng, G., Zhang, Y., et al. 2019. Can biotechnology strategies effectively manage environmental (micro) plastics? Science of the Total Environment 697: 134200.
Kale, S.K., Deshmukh, A.G., Dudhare, M.S., et al. 2015. Microbial degradation of plastic: A review. Journal of Biochemical Technology 6 (2): 952–961.
Paço, A., Duarte, K., da Costa, J.P., et al. 2017. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Science of the Total Environment 586: 10–15.
Espinosa, M.J.C., Blanco, A.C., Schmidgall, T., et al. 2020. Toward biorecycling: Isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Frontiers in Microbiology 11: 404.
Tournier, V., Topham, C., Gilles, A., et al. 2020. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 580 (7802): 216–219.
Kim, H.R., Lee, H.M., Yu, H.C., et al. 2020. Biodegradation of polystyrene by Pseudomonas sp. isolated from the gut of superworms (larvae of Zophobas atratus). Environmental Science and Technology 54 (11): 6987–6996.
Drzyzga, O., and Prieto, A. 2019. Plastic waste management, a matter for the ‘community’. Microbial Biotechnology 12 (1): 66.
Yamamoto, T., Yasuhara, A., Shiraishi, H., et al. 2001. Bisphenol A in hazardous waste landfill leachates. Chemosphere 42 (4): 415–418.
Dauvergne, P. 2018. Why is the global governance of plastic failing the oceans? Global Environmental Change 51: 22–31.
Haward, M. 2018. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nature Communications 9 (1): 1–3.
Manchala, M., and Ramana, B.V. 2020. Beat the plastic pollution. International Journal of Environmental Planning and Development 6 (2): 1–6.
Sidhu, B.K., and Desa, B.H. 2018. Plastics pollution: A new common concern of humankind? Environmental Policy and Law 48 (5): 252–255.
Jalil, M.A., Mian, M.N., and Rahman, M.K. 2013. Using plastic bags and its damaging impact on environment and agriculture: An alternative proposal. International Journal of Learning and Development 3 (4): 1–14.
Singh, P., and Verma, R. 2020. Bioplastics: A green approach toward sustainable environment environmental microbiology and biotechnology, pp. 35–53. Berlin, Germany: Springer.
Burlakovs, J., Kriipsalu, M., Porshnov, D., et al. 2019. Gateway of landfilled plastic waste towards circular economy in Europe. Separations 6 (2): 25.
Žmak, I., and Hartmann, C. 2017. Current state of the plastic waste recycling system in the European Union and in Germany. Tehnički Glasnik 11 (3): 138–142.
Guest, H., Lotze, H.K., and Wallace, D. 2015. Youth and the sea: Ocean literacy in Nova Scotia, Canada. Marine Policy 58: 98–107.
Heidbreder, L.M., Bablok, I., Drews, S., et al. 2019. Tackling the plastic problem: A review on perceptions, behaviors, and interventions. Science of the Total Environment 668: 1077–1093.
Singh, K.D., and Mathur, A. 2019. Plastic pollution in India: An evaluation of public awareness and consumption behaviour. OIDA International Journal of Sustainable Development 12 (07): 25–40.
Wath, S.B., Dutt, P., and Chakrabarti, T. 2011. E-waste scenario in India, its management and implications. Environmental Monitoring and Assessment 172 (1): 249–262.
Brooks, A.L., Wang, S., and Jambeck, J.R. 2018. The Chinese import ban and its impact on global plastic waste trade. Science Advances 4 (6): eaat0131.
Santoleri, J.J., Reynolds, J., and Theodore, L. 2000. Introduction to hazardous waste incineration. New York, NY, USA: John Wiley & Sons.
Yang, Z., Lü, F., Zhang, H., et al. 2021. Is incineration the terminator of plastics and microplastics? Journal of Hazardous Materials 401: 123429.
Ágnes, N., and Rajmund, K. 2016. The environmental impact of plastic waste incineration. Academic and Applied Research in Military and Public Management Science 15 (3): 231–237.
Courtemanche, B., and Levendis, Y.A. 1998. A laboratory study on the NO, NO2, SO2, CO and CO2 emissions from the combustion of pulverized coal, municipal waste plastics and tires. Fuel 77 (3): 183–196.
Huang, D.-Y., Zhou, S.-G., Hong, W., et al. 2013. Pollution characteristics of volatile organic compounds, polycyclic aromatic hydrocarbons and phthalate esters emitted from plastic wastes recycling granulation plants in Xingtan Town, South China. Atmospheric Environment 71: 327–334.
Li, C.-T., Zhuang, H.-K., Hsieh, L.-T., et al. 2001. PAH emission from the incineration of three plastic wastes. Environment International 27 (1): 61–67.
Linak, W.P., and Wendt, J.O. 1993. Toxic metal emissions from incineration: Mechanisms and control. Progress in Energy and Combustion Science 19 (2): 145–185.
Bora, R.R., Wang, R., and You, F. 2020. Waste polypropylene plastic recycling toward climate change mitigation and circular economy: Energy, environmental, and technoeconomic perspectives. ACS Sustainable Chemistry and Engineering 8 (43): 16350–16363.
Yang, H. 2021. Analysis of the advantages and disadvantages of waste incineration and discussion on the standard of incineration. Solid State Technology 64 (2): 6415–6421.
Xiao-Yin, Y. 2003. Disadvantages of waste incineration technology and study on decoupling incineration technology. Environmental Protection Science.
Siddique, R., Khatib, J., and Kaur, I. 2008. Use of recycled plastic in concrete: A review. Waste Management 28 (10): 1835–1852.
White, P.R., Franke, M., and Hindle, P. 1995. Integrated solid waste management: A lifecycle inventory. Berlin, Germany: Springer.
Puri, H.S., Mishra, D.S., and Jindal, V. 2019. Plastic waste management-issues solutions and case studies, 105–113. New Delhi, India: Ministry of Housing and Urban Affairs Government of India.
Dan, C., Xingyuan, H., Peng, W., et al. 2012. Recycling effective ways of waste plastics. Engineering Plastics Application 40: 92–94.
Thomas, C., and Sharp, V. 2013. Understanding the normalisation of recycling behaviour and its implications for other pro-environmental behaviours: A review of social norms and recycling. Resources, Conservation and Recycling 79: 11–20.
Tian, S., Tang, H., Wang, Q., et al. 2021. Evaluation and optimization of blanket production from recycled polyethylene terephthalate based on the coordination of environment, economy, and society. Science of the Total Environment 772: 145049.
Walker, T., Gramlich, D., and Dumont-Bergeron, A. 2020. The case for a plastic tax: A review of its benefits and disadvantages within a circular economy. In Sustainability. Bingley, UK: Emerald Insight. https://doi.org/10.1108/S2514-175920200000004010.
Ahmed, S., Mahmood, Q., Elahi, N., et al. 2020. Current practices and futuristic options in plastic waste management in Pakistan. Central Asian Journal of Environmental Science and Technology Innovation 1 (4): 237–244.
Chowdhury, A.H., Mohammad, N., Haque, M.R.U., et al. 2014. Developing 3Rs (reduce, reuse and recycle) strategy for waste management in the urban areas of Bangladesh: Socioeconomic and climate adoption mitigation option. IOSR Journal of Environmental Science, Toxicology and Food Technology 8 (5): 9–18.
David, A., Thangavel, Y.D., and Sankriti, R. 2019. Recover, recycle and reuse: An efficient way to reduce the waste. International Journal of Mechanical and Production Engineering Research and Development (IJMPERD) 9: 31–42.
Prakash, G., and Pathak, P. 2017. Intention to buy eco-friendly packaged products among young consumers of India: A study on developing nation. Journal of Cleaner Production 141: 385–393.
Karpagavinayagam, P., Rajam, J.A., Suneetha, R.B., Vedhi, C. Prospects of carbon nanomaterial-based sensors for sustainable future. In Carbon nanomaterials-based sensors, pp. 417–428. Amsterdam, the Netherlands: Elsevier.
Bhattacharya, R., Chandrasekhar, K., Roy, P., et al. 2018. Challenges and opportunities: Plastic waste management in India.
Bura, N. 2019. An overview of plastic waste management in India. In Waste management and resource efficiency, pp. 935–943. Singapore: Springer.
Kamali, A.R., Yang, J., and Sun, Q. 2019. Molten salt conversion of polyethylene terephthalate waste into graphene nanostructures with high surface area and ultra-high electrical conductivity. Applied Surface Science 476: 539–551.
Kamali, A.R., and Yang, J. 2020. Effect of molten salts on the structure, morphology and electrical conductivity of PET-derived carbon nanostructures. Polymer Degradation and Stability 177: 109184.
Dai, L., Karakas, O., Cheng, Y., et al. 2022. A review on carbon materials production from plastic wastes. Chemical Engineering Journal 12: 139725.
Putra, P.H., Rozali, S., Patah, M.F., et al. 2022. A review of microwave pyrolysis as a sustainable plastic waste management technique. Journal of Environmental Management 1 (303): 114240.
Bazargan, A., and McKay, G. 2012. A review–synthesis of carbon nanotubes from plastic wastes. Chemical Engineering Journal 195: 377–391.
Vieira, O., Ribeiro, R.S., de Tuesta, J.L.D., et al. 2022. A systematic literature review on the conversion of plastic wastes into valuable 2D graphene-based materials. Chemical Engineering Journal 428: 131399.
Zhang, H., Zhou, X.L., Shao, L.M., et al. 2019. Hierarchical porous carbon spheres from low-density polyethylene for high-performance supercapacitors. ACS Sustainable Chemistry and Engineering 7: 3801–3810.
Gong, J., Michalkiewicz, B., Chen, X., et al. 2014. Sustainable conversion of mixed plastics into porous carbon nanosheets with high performances in uptake of carbon dioxide and storage of hydrogen. ACS Sustainable Chemistry and Engineering 2: 2837–2844.
Liu, X., Ma, C., Wen, Y., et al. 2021. Highly efficient conversion of waste plastic into thin carbon nanosheets for superior capacitive energy storage. Carbon 171: 819–828.
Kaur, B., Gupta, R.K., and Bhunia, H. 2019. Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study. Microporous and Mesoporous Materials 282: 146–158.
Mendoza-Carrasco, R., Cuerda-Correa, E.M., Alexandre-Franco, M.F., et al. 2016. Preparation of high-quality activated carbon from polyethyleneterephthalate (PET) bottle waste. Its use in the removal of pollutants in aqueous solution. Journal of Environmental Management 181: 522–535.
Lazarevic, D., Aoustin, E., Buclet, N., et al. 2010. Plastic waste management in the context of a European recycling society: Comparing results and uncertainties in a life cycle perspective. Resources, Conservation and Recycling 55 (2): 246–259.
Simoneit, B.R., Medeiros, P.M., and Didyk, B.M. 2005. Combustion products of plastics as indicators for refuse burning in the atmosphere. Environmental Science and Technology 39 (18): 6961–6970.
Kumar, A., Dash, S.K., Ahamed, M.S., et al. 2020. Study on conversion techniques of alternative fuels from waste plastics. In Energy recovery processes from wastes, pp. 213–224. Singapore: Springer.
Sharuddin, S.D.A., Abnisa, F., Daud, W.M.A.W., et al. 2017. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Conversion and Management 148: 925–934.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest regarding the publication of this work. In addition, ethical issues including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, and redundancy have been completely witnessed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, P., Dhiman, M., Kansal, A. et al. Plastic waste management for sustainable environment: techniques and approaches. Waste Dispos. Sustain. Energy 5, 205–222 (2023). https://doi.org/10.1007/s42768-023-00134-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-023-00134-6