Abstract
Purpose of Review
The ubiquity of soil contamination by lead (Pb) and arsenic (As) has prompted the development of numerous techniques for its remediation. For human health exposure assessment, oral bioavailability-based methods are the most suitable to assess the efficacy of these treatment strategies, including in vivo relative bioavailability (systemic absorption relative to a toxicity reference) and in vitro bioaccessibility (dissolution in simulated gastrointestinal solutions). This paper provides a critical review of opportunities and challenges associated with the immobilization of Pb and As in contaminated soil.
Recent Findings
This review identified that the major inorganic and organic amendments used to reduce Pb and As exposure include phosphate, industrial by-products, metal oxides, organic matter, biochar, and treatment with iron sulphate to promote the formation of plumbojarosite in soil. In addition to RBA and IVBA assessment, investigating changes in Pb/As speciation in untreated vs treated soil can provide additional confirmation of treatment efficacy. The results of this review showed that immobilization efficacy may vary depending on amendment type, Pb, and As speciation in soil and the approach used for its assessment.
Summary
Reducing childhood exposure to Pb and As is a significant challenge, given the variety of contamination sources and treatment strategies. A lines-of-evidence approach using standardized methodologies is recommended for the assessment of immobilization efficacy to ensure exposure and risk reduction
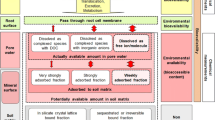
Graphical Abstract
Bioavailability-based remediation strategies. Popular soil amendments to reduce Pb exposure include phosphate, industrial by-products, metal oxides, organic matter, and biochar; however, these may increase As exposure. The plumbojarosite formation technique has been recently developed to mitigate Pb and As exposure simultaneously. Multiple lines-of-evidence approach is recommended to assess treatment efficacy

Similar content being viewed by others
Introduction
Widely accepted techniques to minimize exposure to lead (Pb) contaminated soil, such as capping or excavation, are expensive, disruptive, and unsustainable. Furthermore, excavation followed by replacement with clean soil requires a source of clean material and a repository for contaminated soil. While these approaches are necessary for highly impacted sites, they are not practical or economically feasible for moderately impacted soil due to their widespread occurrence. These limitations have necessitated the development of alternative risk management strategies that reduce exposure and toxicity of contaminants left in situ by chemical immobilization utilizing soil amendments [1, 2].
Over the past 20 years, many studies have focused on Pb immobilization due to its ubiquitous distribution in the environment and potential adverse health effects associated with exposure. Incidental ingestion is the highest-risk exposure pathway to Pb from contaminated soils [1]. Treatment effectiveness is determined by reduction in contaminant bioavailability, as measured by uptake of the contaminant in systemic circulation in vivo after animal feeding exposure, or bioaccessibility, as measured by contaminant extractions using simulated gastrointestinal solutions in vitro. Discussions on immobilization mechanisms were considered out of scope for this review because several reviews already exist which have focused on this topic [3,4,5, 6•, 7], and the readers are directed to these reviews and references therein for further information. Similarly, effects on leachability (e.g., using ammonium acetate extraction) and impacts on ecological receptors (e.g., plants and earthworms) have also been reviewed recently and were not considered during this review [6•, 8]. However, few reviews have assessed Pb immobilization efficacy using a bioaccessibility/bioavailability reduction approach, although this approach may be considered the most relevant to understand the impact of Pb immobilization strategies on human health. Therefore, this review highlights recent studies (2014–2022) on Pb immobilization strategies on human health exposure with a focus on in vivo bioavailability and in vitro bioaccessibility.
Lead-contaminated soil is often impacted by inorganic co-contaminants, particularly arsenic (As). Owing to the antagonistic impact of several Pb immobilization strategies on As immobilization [6•], the applicability of these strategies in co-contaminated soil remains debatable. Therefore, among the Pb immobilization studies reviewed here, As bioavailability/bioaccessibility results (when available) were also summarized to provide a more holistic assessment of treatment strategies for soils contaminated by both Pb and As. Specifically, we aim to (1) review studies using soil amendments as immobilization techniques and best practice methods to assess treatment effectiveness, (2) compile and compare available research to show the distribution of treatment efficacy results by soil amendment type, and (3) include both Pb and As data when present as co-contaminants because of the frequency of co-contamination and known antagonistic side effects between Pb and As following few treatments.
Immobilization Strategies for Reducing Pb and As Exposure from Contaminated Soil
Soils differ in their chemical, physical, and biological properties with parameters such as pH, redox potential, organic matter, clay content/type influencing contaminant solubility, bioavailability, and exposure for ecological and human receptors. Soil pH has the potential to modify metal solubility/availability by controlling dissolution/precipitation and by regulating the ionization of pH-dependent ion exchange sites on organic matter and permanently/variably charged clay minerals. The ionization of pH-dependent functional groups on soil organic matter also affects the formation of stable organometallic complexes [9, 10]. Soil chemical reactions between clay mineral surfaces and Pb are primarily attributed to the adsorption of Pb to cation exchange sites or specific adsorption of Pb to oxyhydroxide surfaces of amorphous Fe, Al, and Mn oxides [11,12,13]
Lead reacts with soil components depending on soil pH to form phases ranging from weakly bound and potentially bioavailable forms [for example, lead oxide (PbO) and lead carbonate (PbCO3)] to strongly bound and non-bioavailable forms [for example, chloropyromorphite (Pb5(PO4)3Cl) and plumbojarosite (PbFe6(SO4)4(OH)12)]. In situ stabilization of soil Pb using amendments focuses on reducing environmental risk by chemically altering potentially bioavailable soil Pb species to less bioavailable forms. Common soil amendments have been used for Pb immobilization to induce the formation of less soluble mineral phases (e.g., phosphate or ferric sulphate amendments) and/or form strong complexes with Pb via inner-sphere mechanisms (e.g., Fe oxides, Mn oxides, biosolids, and compost). Additionally, sources such as biosolids and compost may facilitate immobilization by inducing Pb phosphate formation in soils.
Similar to Pb, soil As can also exist in a wide variety of forms that have a wide range of solubilities. Arsenic typically exists in soils in the (III) and (V) oxidation states and may be present in the (–III) and (0) oxidation states in strongly reduced soils and sediments. Arsenic in the (V) form, arsenate, is stable in aerobic soils. Unlike Pb, As in soil solution exists mainly as an oxyanion and its sorption decreases with increasing soil pH and in the presence of competing anions, such as orthophosphates. Arsenic is often associated with Fe oxides in soil but can also be adsorbed to CaCO3 surfaces in calcareous soils. Arsenic sorbed to CaCO3 is easily dissolved under acidic conditions of the gastrointestinal tract and becomes potentially bioavailable to humans whereas As sorbed to Fe oxide may not readily dissolve in the gastrointestinal track and will potentially be less bioavailable [14]. Soil amendments for As immobilization typically involve the application of Fe or Fe oxide to soil to bind As as either insoluble minerals or strong complexes [15]. Once stabilization is achieved via chemical alteration, the next challenge is assessing the resulting changes in human health and environmental risk associated with soil Pb/As [14].
Approaches to Determine the Efficacy of Remediation Strategies
Assessment of Pb and As Exposure Reduction Using Bioavailability/Bioaccessibility-Based Methods
Assessment of bioaccessibility and bioavailability in pre- and post-remediated soils inform the efficacy of clean-up efforts to reduce human health exposure in heterogenous soil systems, which in turn, facilitates efficient and cost-effective remediation of contaminated soil. Bioavailability measurements using in vivo animal models provide the best approach for assessing Pb/As exposure following incidental ingestion of contaminated soil. This determination is especially crucial as an efficacy metric when designing immobilization strategies for soil co-contaminated with Pb and As [16, 17, 18••, 19••, 20]. Absolute bioavailability, the fraction of an ingested contaminant that crosses the gastrointestinal epithelium and is internally distributed [16], may be challenging to determine; therefore, relative bioavailability (RBA) is calculated as the ratio of contaminant retained by an in vivo model upon ingestion of contaminated soil compared to that of a standard soluble contaminant assumed to be 100% bioavailable (e.g., Pb acetate or sodium arsenate) [19••, 21, 22, 23••]. Contaminant RBA has been determined using animal models including mouse, swine, and monkeys [21, 22, 24,25,26]; however, mouse and swine are most commonly utilized to evaluate Pb/As immobilization techniques [17, 20, 27,28,29].
Although animal models are critical for assessing immobilization efficacy, expense and ethical considerations can limit access to RBA measurement [30,31,32,33]. To overcome this limitation, RBA may be predicted using in vitro bioaccessibility (IVBA) assays, designed to simulate the dissolution of soil Pb/As in the gastrointestinal tract [16, 23••, 34, 35]. Over the past 25 years, various IVBA methods have been developed to estimate RBA through in vivo in vitro correlations (IVIVC), for example, USEPA Method 1340, Simplified Bioaccessibility Extraction Test (SBET), Physiologically Based Extraction Test (PBET), Solubility Bioaccessibility Research Consortium (SBRC), Unified Bioaccessibility Research Group of Europe Method (UBM), In Vitro Gastrointestinal Extraction Method (IVG), and Deutsches Institut für Normunge.V. (DIN). Although further investigations are needed to validate IVIVCs for soil post-immobilization [36, 37], the use of reliable IVBA assays for heterogenous soil systems is likely the best approach to lessen the need for widespread RBA measurement.
Assessment of Changes in Pb and As Speciation
The environmental and toxicological fate of Pb/As is linked to their speciation as different forms behave differently in environmental and biological systems. Few speciation endpoints may be stable under environmental conditions (e.g., mineral sorbed phases); however, these same forms may be unstable when they enter the low pH stomach environment following ingestion, resulting in potential biological uptake. Conversely, other Pb/As forms (e.g., pyromorphite, plumbojarosite, beaudanite, segnitite) exhibit environmental and biological stability and are the favored endpoints for immobilization strategies. Therefore, an understanding of elemental speciation in amended soil can gauge both the environmental and toxicological fate, as well as identify forms that significantly reduce the burden of environmental risk. Demonstrating the formation of species with low bioavailability characteristics, resulting from the immobilization strategy, is an important line-of-evidence approach.
A limited number of analytical techniques have been used to speciate Pb/As in environmental samples, each with their benefits and limitations. A detailed review of all mechanisms of speciation was considered out of scope for this review, and readers are referred to previous reviews for metal speciation methods in soils, such as Tack and Verloo [38] and D’Amore et al. [2]. Based on the speciation methods used in the 39 articles included in this review, sequential extraction method, X-ray diffraction (XRD), scanning electron microscopy with energy dispersive X-ray analysis (SEM–EDX), and X-ray absorption spectroscopy (XAS) were discussed in terms of benefits and limitations of each method.
Sequential extraction methods have been widely used and reported due to their ease of use, low analytical requirements, and useful information on solubility [39,40,41,42]. However, metal speciation can be altered during the extraction procedure between sequential steps, casting doubt on the accuracy of this method for speciating metals [43,44,45]. Interpretation of results should be qualified that they are metal solubility fractions, functionally defined by the extraction method, and not a true and direct measure of speciation.
Crystalline phases of Pb and As can be identified in samples using XRD; however, XRD has a high limit of detection (~ 2% wt.). Additionally, XRD is only able to measure crystalline phases, not adsorbed or poorly crystalline phases, which are common in environmental media. Microscopy-based speciation methods such as SEM–EDX have been used to determine total Pb/As concentration in discrete particles to support species identification. However, the sample size which can be measured is in the order of individual particle, so this method is limited for representability in heterogenous environmental samples. In addition, the interpretation of speciation by the relative abundance of elemental fluorescence is indirect, as it provides no information to support bonding types or oxidation state, suffers from assumptions and bias, and is surface sensitive.
XAS can be considered a superior tool to identify Pb/As speciation in environmental samples. The use of XAS for accurate speciation of metals in soils has been previously reviewed by Gräfe et al. [46]. This technique measures the atomic bonding environment of the target element. This can be semi-quantitatively fit by statistical linear combination fitting (LCF) of standards to yield relative abundances of metal species ranging from fully hydrated dissolved ions to crystalline minerals. When assessing soil amendment immobilization efficacy, XAS has been used to determine the conversion of Pb/As to low bioavailability phases providing insight into the expected bioavailability of Pb/As in soil pre- and post-treatment [12, 13, 17, 18••, 19••, 37, 47,48,49,50]. Directly linking RBA of LCF components (Pb/As standards) to speciation results for unknown soils has the potential to be an effective tool for estimating contaminant bioavailability and immobilization efficacy [18••, 19••]. While XAS is the preferred method for the identification and semi-quantification of adsorbed, poorly ordered, and crystalline Pb/As phases in contaminated soil, the technique has challenges and limitations. XAS assessment is not routine as it requires highly trained analysts and is difficult to access experimental beamtime. In addition, data analysis and interpretation are highly dependent on reference materials used, and the technique has a relative quantification error of ~ 10%. Although soil Pb/As speciation greatly impacts RBA, speciation results may not always explain the observed RBA. Factors affecting RBA beyond speciation include the presence of competing cation/oxyanions, organic matter content, soil pH, and physical characteristics (e.g., specific surface area and surface passivation) also account for variability in immobilization efficacy [7, 12, 16, 20, 21, 23••, 29, 44].
To summarize, RBA paired with validated IVBA assays remains the most effective methodology for assessing soil Pb/As immobilization efficacy. While there are constraints to performing bioavailability analyses, a combination of IVBA assays and XAS techniques provides effective windows into biogeochemical drivers of immobilization strategies. This is especially the case for amendments promoting mineral phases with exceptionally low solubility at acidic conditions and possessing distinct spectral characteristics (i.e., jarosite-group minerals). The use of validated IVBA assays allows for a more widespread assessment of amendment applications in heterogenous soils, while bone slope ratio using the mouse model allows for increased time and cost efficiency when RBA is needed, thereby requiring less in vivo research without compromising data quality.
Impact of Soil Amendments on Pb and As Relative Bioavailability
Peer-reviewed research articles published after 2013 were retrieved from two databases: Scopus and Web of Science. Keywords used during the search were “lead AND remediation,” “lead AND immobilization,” “lead AND soil,” “lead AND phosphorus,” “lead AND compost,” and “lead AND oxides.” A total of 299 articles were retrieved during this search from which duplicates were removed and articles where soils were spiked with Pb/As for < 2 months were removed. The remaining articles were screened for use of “in vivo relative bioavailability” or “in vitro bioaccessibility” to assess treatment efficacy. After screening, 39 articles were selected to be included in this review.
Data regarding Pb treatment efficacy (and As treatment efficacy where available) were retrieved from these 39 articles and converted to TER using Eq. 1.
A summary of these studies comparing treatment effect ratios (TERs) for each amendment type is given using in vivo (Fig. 1) and in vitro (Fig. 2) approaches. Where present, the impact on As RBA/IVBA immobilization efficacy using TER was also included in the figures.
Lead (Pb; Fig. 1A) and arsenic (As; Fig. 1B) immobilization efficacy following treatment of contaminated soil with various amendments [17, 18••, 19••, 36, 37, 48, 56, 60]. Immobilization efficacy was determined using in vivo relative bioavailability (RBA) with treatment effect ratio (TER) calculated by dividing Pb/As RBA in treated soil by Pb/As RBA in untreated soil. A ratio of 1 indicates no immobilization effect while values < 1 denote that immobilization is effective, with smaller TER indicating greater immobilization efficacy. Box plots are presented for Pb TER data with the total number of data points per treatment category provided above the maximum value. Arsenic TER is presented as a single data point as its immobilization efficacy in Pb/As co-contaminated soil has only been assessed using RBA in one study
Lead (Pb; 2a, b) and arsenic (As; 2c, d) immobilization efficacy following treatment of contaminated soil with various amendments [13, 18••, 36, 37, 48, 54,55,56,57,58••, 59, 60,61,62, 64, 66, 67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. Immobilization efficacy was determined using in vitro bioaccessibility (IVBA) with treatment effect ratio (TER) calculated by dividing Pb/As IVBA in treated soil by Pb/As IVBA in untreated soil. A ratio of 1 indicates no immobilization effect while values < 1 denote that immobilization is effective, with smaller TER indicating greater immobilization efficacy. TERs > 1 indicate contaminant mobilization where the application of amendment strategies increased Pb/As IVBA compared to unamended soil. TERs were determined following IVBA assessment using gastric phase (Fig. 2a and c; pH 1.5–2.5) and intestinal phase extraction (Fig. 2b and d; pH 6.5–7.0). Box plots are presented for Pb/As TER data with the total number of data points per treatment category provided above the maximum value
A TER of 1 indicates no immobilization effect while values < 1 denote that immobilization is effective, with a smaller TER indicating greater immobilization efficacy. TERs > 1 indicate that application of amendment strategies increased Pb/As RBA/IVBA (contaminant mobilization) compared to unamended soil. It is acknowledged that TERs are influenced by the physicochemical properties of individual amendments within each of these categories, in addition to application rates, soil characteristics, and contaminant properties. Similarly, TERs may be influenced by differences between in vivo and in vitro operational parameters, which impact RBA/IVBA outcomes. Soil amendment strategies presented in Figs. 1 and 2 have been broadly classified into different categories and discussed below.
Phosphate Amendments
Immobilization Efficacy Assessed Using RBA
Despite being the most frequently used amendment strategy for Pb-contaminated soil, a wide range of TERs using RBA have been reported for phosphate amendments (0.03–0.90). Variability in TERs may arise from the use of different phosphate types with varying solubility, including phosphoric acid (PA), rock phosphate (RP), monocalcium phosphate (MCP), single or triple superphosphate (SSP/TSP), mono/di ammonium phosphate (MAP/DAP), calcium hydrogen phosphate (CaHPO4), struvite (MgNH4PO4.6H2O), and potassium phosphate (KH2PO4). A recent meta-analysis review of phosphate amendment studies [51] identified that soluble amendments such as PA showed higher immobilization efficacy than insoluble amendments such as RP. TERs may also vary due to differences in application rates (0.5–10% w/w), P:Pb molar ratios (1:1–5:1), the presence of other soil constituents (e.g., Ca and Zn), which may interact with phosphate, contamination source and the initial concentration of Pb in soils (i.e., highly contaminated mine impacted soils vs. low/mild elevation as in most urban soils). When assessed using an in vivo approach, TERs for phosphate-amended Pb-contaminated soil have ranged from 0.03 to 0.90. These studies include a long-term Pb immobilization investigation [17] where phosphate (TSP, 1% P) ± Fe (2.5%)/biosolid (10%) amendments resulted in TERs of 0.25–0.69. XAS analysis revealed that Pb phases in untreated soil included galena (PbS), anglesite (PbSO4), adsorbed, and organic-bound Pb. However, following treatment, the predominant Pb species were sparingly soluble pyromorphites and Pb phosphates, with a significant reduction in highly bioavailable anglesite. In another study [47], soils impacted by shooting range activities, smelting, or non-ferrous slag application were treated with PA and RP (P:Pb = 5:1), resulting in TERs of 0.03–0.90. For each soil, the greatest reduction in Pb RBA was observed for PA amendments where 43–67% of Pb was transformed to pyromorphite. However, similar TERs were reported when PA or RP amendments were provided orally to mice suggesting that interactions between Pb and phosphate in situ are not a prerequisite for RBA reduction, rather this may occur fortuitously in vivo if Pb and phosphate are ingested concurrently.
Although phosphate amendments have the potential to immobilize Pb, their field application in As-co-contaminated soil may be compromised due to the ability of phosphate to displace As from soil binding sites. Conceivably, this may result in an increase in As mobilization and TERs > 1, although only one study out of the 39 reviewed here has assessed this. Following treatment with PA in Li et al. [56], no change in As RBA was observed in two soils (TER = 1.04 and 1.05), decreased As RBA occurred in two soils (TER = 0.58 and 0.80) and increased As RBA in another (TER = 1.54) [56]. The variable As RBA results may be attributable to differences in soil phosphate availability following interactions with Pb and other soil constituents. Because phosphate may displace As from soil binding sites, the fraction of As available for absorption into the systemic circulation may increase following soil phosphate treatment. However, studies have shown that inorganic arsenate (AsV) is absorbed via intestinal epithelium phosphate transporters with phosphate outcompeting AsV for absorption sites [52]. Because AsV is a predominant As species in aerobic soil, it is reasonable to consider that phosphate will compete with As for transepithelial transport. The net effect of phosphate amendments on As RBA may be a balance between the role of phosphate in increasing As solubility in soil and decreasing bioavailability in vivo because of competition between phosphate and As for absorption across the gut epithelium. Studies assessing the immobilization effects of phosphates towards As are mostly based on IVBA assays (Fig. 2); additional in vivo studies are required to ascertain treatment efficacy in Pb/As co-contaminated soils.
Immobilization Efficacy Assessed Using IVBA
When assessed using an in vitro approach, Pb TERs for phosphate-amended soil ranged from 0.11 to 1.8 (Fig. 2a and b). The most widely used IVBA approach for assessing treatment efficacy was USEPA Method 1340 [also known as Relative Bioaccessibility Leaching Procedure (RBALP), SBRC assay gastric phase, and SBET assay]. Although this method has been validated for use to assess Pb RBA in contaminated soil [53], its suitability for assessing treatment efficacy in phosphate-amended soil is debatable. Obrycki et al. [54] contended that USEPA Method 1340 overestimates Pb IVBA owing to the strong acidic conditions of the gastric phase (pH 1.5), which influences Pb solubility. As a consequence, phosphate amendments appear to be “largely ineffective” in immobilizing Pb when assessed using this assay. To overcome this issue, Obrycki et al. [54] modified the IVBA assay pH to 2.5 and excluded glycine, which resulted in a significant negative correlation between Pb IVBA and the amount of Pb phosphate in treated soil (r2: 0.42, p < 0.001). In studies where assays were conducted at pH 1.5 and 2.5 for the same treated soils [36, 54,55,56], considerably lower Pb TERs were observed at pH 2.5 compared to 1.5. However, Scheckel et al. [7] cautioned against the use of IVBA assessment at a pH > 1.5 to predict Pb immobilization efficacy because of the potential of assay artifacts (i.e., formation of Pb phosphates in vitro at higher pH), thereby overpredicting treatment efficacy. In addition, as an IVIVC is yet to be established for Pb RBA-IVBA using an IVBA approach at pH 2.5 without glycine, it is unclear whether the relationship reported in Obrycki et al. [54] is appropriate for predicting changes in Pb RBA in phosphate amended soils. In vitro assessment of Pb immobilization efficacy has also been undertaken using the intestinal phase of IVBA assays (pH 5.5–7.5; Fig. 2). Although in vitro Pb phosphate formation may occur when the gastric phase is transitioned to the intestinal phase (i.e., the interaction of soluble Pb and P at pH > 1.5), as previously mentioned, a similar phenomenon was observed in vivo where sequential administration of Pb-contaminated soil and phosphate in mice resulted in significant reductions in Pb RBA (TERs of 0.03–0.90) as a result of Pb phosphate formation [36].
In Pb/As co-contaminated soil, variable As TERs (0.34–16.0) have been reported when soils treated with phosphate amendments were assessed using IVBA approaches. For example, Li et al. [56] reported As TERs of 1.09–12.0 when PA (0.5%) amended soils were assessed using USEPA Method 1340 (pH 1.5). In addition, higher TERs (1.37–16.0) were observed when gastric conditions were modified to the intestinal phase (pH 7.0). Similarly, Garau et al. [57] showed that the addition of a municipal solid waste compost (MSWC) high in phosphate to a mining-impacted soil (48.8 mg As kg–1) significantly increased intestinal phase As IVBA (TER = 10.6) after 4 months of aging. After the addition of 2% and 4% MSWC, available phosphate in soil increased from 22.6 to 32.8–44.8 mg kg–1. The higher amount of phosphate enhanced As release from soil adsorption sites, thereby leading to significantly higher intestinal phases As IVBA. However, it is noteworthy that in vivo effects of phosphate on As RBA may be significantly different from in vitro effects, since phosphate may decrease As absorption from the gastrointestinal tract via phosphate transporters. Further in vivo assessment and the establishment of IVIVC are needed to elucidate the effects of phosphate amendments on As RBA.
Although phosphate amendments may exert opposing effects on Pb/As IVBA, recent studies have proposed novel approaches to reduce both Pb/As IVBA simultaneously. A practical solution to the antagonistic behavior between phosphate and Pb/As was suggested by Rathnayake and Schwab [58••], where sequential additions of phosphate (P:Pb = 5:1), followed by Fe-rich water treatment residues (WTRs; Fe:As = 10:1) substantially reduced both Pb and As IVBA (TERs of 0.50 and 0.34 respectively). Similarly, Liao et al. [59] suggested the use of a hybrid iron, sulfate, and phosphate-based bio-nanocomposite consisting of nano hydroxy ferric phosphate and hydroxy ferric sulfate particles coated on Aspergillus niger. The material was used to simultaneously immobilize As, Cd, and Pb in soil impacted by Pb–Zn smelting. After 60-d aging, based on USEPA Method 1340 with the inclusion of 0.5 mL L–1 acetic acid, As, Cd, and Pb TERs were 0.80, 0.60, and 0.72, respectively, suggesting the potential of composite amendments for simultaneously immobilizing co-contaminants in situ.
Industrial By-Products
Immobilization Efficacy Assessed Using RBA
Two studies have used an RBA approach to assess the Pb immobilization potential of Al/Fe-rich WTRs (with and without phosphate amendments), with TERs ranging from 0.45 to 0.74. Mele et al. [60] used either Al or Fe-rich WTRs containing amorphous Al/Fe/Mn hydroxides and high organic matter content (14.5–24.1%). A significant reduction in Pb RBA was observed after the application of 2% w/w Al and Fe-rich WTR amendments (TERs of 0.74 and 0.52 respectively), which was attributed to Pb co-precipitation with Fe during transit from the stomach to the small intestine, or competition between Fe and Pb for absorption via divalent metal transporter 1 (DMT-1). Using Fe-rich industrial waste (2.5% Fe) + TSP (1% P), Bradham et al. [17] reported Pb TERs of 0.45–0.53, which were lower than TERs for PA only (1% P) and TSP only (3.2% P) treated soils (TERs of 0.52–0.67 and 0.54–0.68 respectively). XAS analysis revealed that Fe/TSP and PA-treated soil contained similar Pb phases, which were dominated by pyromorphite and tri-Pb diphosphate (Pb3(PO4)2), with minor phases of adsorbed/organic bound Pb and anglesite. The increased efficacy due to the addition of Fe was attributed to Pb adsorption/incorporation into Fe (hydr)oxides, as well as increased competition between Pb and Fe for transport across DMT-1.
Immobilization Efficacy Assessed Using IVBA
High Pb TERs have been observed following the use of Al/Fe-rich WTR amendments, with Pb TERs ranging from 0.93 to 0.99 in Silvetti et al. [61], 0.93 to 0.94 in Mele et al. [60], and 0.79 to 0.96 in Rathnayake and Schwab [58]. However, Rathnayake and Schwab [58••] reported a TER of 0.26 when using Fe-rich WTRs (Fe:Pb = 10:1) and phosphate (P:Pb = 5:1) simultaneously (using USEPA Method 1340) compared to Fe-rich WTRs alone (TER of 0.96), phosphate alone (TER of 0.50), or sequential application of phosphate followed by Fe-rich WTRs (TER of 0.50). This result suggests that simultaneous additions of phosphate and Fe may enhance Pb immobilization efficacy by converting Pb species into pyromorphite or causing Pb to be adsorbed/incorporated into Fe (hydr)oxides. However, as highlighted by Mele et al. [60], lower TERs were observed for Al or Fe-rich WTR-treated soil when assessed using RBA compared to IVBA approaches, as a result of competition between Fe and Pb for absorption. Therefore, in addition to using IVBA to screen Pb immobilization treatment efficacy of Al/Fe rich WTR amendments, using RBA may be necessary to confirm TER in vivo as a line of evidence approach for this amendment type.
Variable effects on As TERs have been observed when Fe/Al-rich WTRs were applied to soil co-contaminated with As. Using USEPA Method 1340, Mele et al. [60] showed that the application of 1 and 2% (w/w) Fe-rich WTRs (0.6% and 2.1% Fe respectively) and Al-rich WTRs (0.6% Fe 3.4% and 9.8% Al respectively) to a mine-impacted soil (2267 mg As kg–1) did not significantly reduce As IVBA (TERs of 0.85–0.95). Similarly, using the SBRC gastric phase, Silvetti et al. [61] showed that treatment of two soils (2428 and 860 mg As kg–1) impacted by mining and chromated copper arsenate with an Al-WTRs (13.9% Al) marginally reduced As IVBA (TERs of 0.80–0.81). In contrast, although Rathnayake and Schwab [58] showed significant reductions in As IVBA (USEPA Method 1340) when Fe-WTRs containing 12% Fe were applied to a smelter-impacted soil (7520 mg As kg–1 and 66,400 mg Pb kg–1) at Fe:As molar ratios of 2:1, 5:1, and 10:1 (TERs of 0.27, 0.06, and 0.04), a significant reduction in simultaneous Pb and As IVBA was only observed at the highest application rate (TER of 0.79). The ineffectiveness of Fe-WTRs in immobilizing Pb compared to As may be due to the fact that Pb adsorbed by Fe oxides is readily solubilized in gastric solution. These results highlight that WTR immobilization efficacies vary due to differences in application rates (2–10%), in addition to varying Fe concentrations (2–12%), and more studies using RBA approaches are necessary to confirm the observed in vitro treatment efficacy in vivo.
Metal Oxides
Immobilization Efficacy Assessed Using RBA
Two studies have assessed the Pb immobilization potential of metal oxides using RBA (TER of 0.54 and 0.90). Utilizing nano-zero valent Fe (nZVI), Mele et al. [60] determined a Pb TER of 0.54, where immobilization was attributed to Pb adsorption/co-precipitation with Fe during transit in the gastrointestinal tract. When assessed in vivo, dissolution of metal oxides in gastric solution may occur; the elevated Fe concentration in the gastrointestinal tract competing with Pb absorption via DMT-1 and reducing Pb RBA. Thus, changes in Pb RBA with metal oxide soil amendments may not only reflect in situ Pb immobilization but also in vivo competition between Fe and Pb for absorption transporters. In contrast, Kastury et al. [48] demonstrated that hematite (Fe2O3) amendments only marginally reduced Pb RBA (TER of 0.90), which was supported by the unaltered distribution of Pb species in untreated and hematite-treated soil. The contrasting results between these two studies demonstrate that nZVI is more efficient at reducing Pb exposure in vivo compared to hematite, conceivably due to stronger adsorption/co-precipitation interactions and/or competition between Pb and nZVI for absorption via DMT-1.
Immobilization Efficacy Assessed Using IVBA
Studies that have assessed Pb immobilization efficacy using IVBA assays following the application of goethite, ferrihydrite, hematite, n/µZVI, and Mg oxide amendments have observed limited success (TERs of 0.68–1.17). Although Pb can be adsorbed to metal oxides in situ when materials are applied to contaminated soil, metal oxides may solubilize in gastric solution, thus releasing adsorbed Pb and resulting in high TER.
Varied immobilization performance has also been observed for metal oxides when applied to As co-contaminated soils. Rathnayake and Schwab [58] applied goethite and ferrihydrite to contaminated soil at Fe:As molar ratios of 2:1, 5:1, and 10:1, which resulted in a decrease in As IVBA (USEPA Method 1340; TERs of 0.54–0.91), showing increasing immobilization efficacy with increasing application rate. However, the performance of Fe oxides was less effective at the same Fe:As molar ratio when compared to Fe-WTRs (TERs of 0.04–0.27). The high immobilization capacity of Fe-WTRs is possibly related to the higher amount of Fe that was available to adsorb As compared to goethite and ferrihydrite applications. In Mele et al. [60], n/µZVI application (1% w/w) to mine-impacted soil was effective at immobilizing As (TERs of 0.59–0.71 based on USEPA Method 1340) although Pb immobilization was ineffective (TERs of 0.98–0.99). A similar result was reported by Nandillon et al. [62] using USEPA Method 1340 following the addition of ZVI (1.5% w/w) to a mining-impacted soil (TERs of 0.49 and 1.04 for As and Pb respectively). The effectiveness of n/µZVI in immobilizing As was reflected by the in situ transformation of As from soluble (hematite sorbed As) to low-solubility species (arsenosiderite) [60] although its limited capacity to decrease Pb IVBA suggests that n/µZVI sorbed Pb is solubilized under gastric phase conditions.
Organic Matter
A variety of organic matter amendments (e.g., biosolids, municipal solid waste/manure compost, peat, sodium humate, and activated charcoal) have been utilized to immobilize Pb/As in soil, although only one study has assessed their efficacy in conjunction with phosphate amendments using RBA approaches. Dissolved organic matter is capable of donating C as an electron donor for reducing arsenate, as well as serve as a complexing agent to which Pb/As may bind and become less bioavailable [63]. Bradham et al. [17] reported Pb TERs of 0.28–0.55 when biosolid (10%) and TSP (1% P) were added to a Pb-contaminated soil. XAS analysis revealed that organic matter-bound Pb was the predominant phase in soil treated with biosolids and TSP, followed by pyromorphite and Pb3(PO4)2, with anglesite and adsorbed Pb as minor constituents.
However, when organic matter amendments were assessed using IVBA approaches, Pb immobilization efficacy has been modest to negligible (TERs of 0.76–1.36). Li et al. [28] suggested that Pb immobilization in pig manure (3–12% w/w) amended soil may occur due to increases in organic C, total N, and available P. Although no speciation analysis was conducted, it is conceivable that similar to studies by Bradham et al. [17], increased organic C and P may have promoted the formation of organic bound Pb and Pb-phosphate minerals, thereby causing a reduction in Pb IVBA. Using XAS analysis, Attanayake et al. [13] also identified increasing organic matter-bound Pb in the field and stability of Fe-bound Pb during PBET extraction as potential contributors to the reduced Pb IVBA in compost and composted and non-composted biosolids amended soils.
In contrast, the application of organic matter has been ineffective at immobilizing As and, in some instances, has enhanced the release of As. Garau et al. [57] reported As TERs > 10 (using the SBRC intestinal phase) when MSWC (2–4% w/w) was added to a Pb/As co-contaminated soil. The authors claimed that the increase in soil pH (5.93 to 6.53–6.78) following the amendment facilitated the release of As from adsorption sites. In addition, as the MSWC was a rich source of available P (62.2 mg kg−1), this may have facilitated the exchange of sorbed AsV, leading to As release.
Biochar and Other Amendments
A variety of biochars, produced from several source materials (manure, wheat straw, rice straw, grand fir shavings), have been assessed for their potential to immobilize Pb/As in contaminated soil. Although in vivo assessment of immobilization efficacy has not been undertaken, in vitro assessment has highlighted the limited capacity of these materials for soil treatment (Pb TERs of 0.66–1.36; As TERs of 0.92–1.27 using USEPA Method 1340). The only exception, reporting high Pb immobilization efficacy was a study by Zhang et al. [64] where rice straw biochar (2.5% w/w) was added to a Pb-contaminated soil (487 mg Pb kg−1) resulted in a TER of 0.26 (PBET-G, pH 2.0). However, following aging (up to 5 years), a reduction in soil pH and organic carbon occurred which decreased immobilization efficacy (i.e., Pb IVBA increased by 9.4% in amended soil), which raised questions regarding the longevity of biochar-mediated Pb immobilization.
Other amendments including red mud, lime, multiwall carbon nanotubes, flue gas desulfurization gypsum, sulfur, and bentonite have been utilized for Pb/As immobilization although only in vitro assessment has been used to determine treatment efficacy. These amendments generally showed low to negligible treatment efficacy (Pb TERs of 0.91–1.03; As TERs of 0.82–1.11 using USEPA method 1340). However, Zhang et al. [64] reported a Pb TER of 0.23 (PBET-G, pH 2.0) following the addition of 2.5% w/w bentonite to acidic Pb-contaminated soil (487 mg Pb kg−1). Zhang et al. [64] attributed Pb immobilization to surface adsorption, the isomorphic substitution of metal ions into the bentonite structure and Pb precipitation facilitated by the increase in soil pH. However, similarly to the above-mentioned biochar results, Pb IVBA increased by 9.5% following aging of bentonite-amended soil.
Plumbojarosite Treatment
Immobilization Efficacy Assessed Using RBA
A recently developed technique for the immobilization of Pb/As in contaminated soil is through the formation of new Pb/As species, following soil treatment with Fe2(SO4)3 (0.1–1.0 M) and H2SO4 (0.01 M) at elevated temperature (> 95 °C). Using a slow addition method (1.8 mL/h) for up to 67 h, Karna et al. [18••] and Sowers et al. [19••] reported low TERs (0.012–0.068) for Pb compounds, Pb-spiked soil, and Pb-contaminated soil, highlighting that the treatment results in the formation of Pb species that exhibit low bioavailability characteristics. Kastury et al. [65••] expanded on the concept and proposed that a solid:solution ratio of 1:1 could be used to minimize water usage with Fe3+ application of 0.4–1.0 M, H2SO4 of 0.05 M, and treatment at 150 °C for 1 h. Using this approach, TERs of 0.01–0.27 were achieved for 4 soils with Pb concentrations ranging from 1500 to 6340 mg kg−1. Both Karna et al. [18••] and Kastury et al. [65••] utilized XAS to compare Pb species before and after treatment. Surface adsorbed Pb phases and PbSO4 were converted to jarosite minerals (plumbojarosite, segnitite, and beaudanite) at high conversion efficiency (93–99%), which exhibited extremely low solubility, especially at acidic pH. In a follow-up study, Sowers et al. [19••] confirmed that plumbojarosite transits through the gastrointestinal tract accounting for its low bioavailability. In addition to exhibiting high Pb immobilization efficacy, Kastury et al. [65••] identified that As is also immobilized during the treatment (TER of 0.70), although this remains to be assessed in additional co-contaminated soils and under more practical field conditions.
Immobilization Efficacy Assessed Using IVBA
Complementary IVBA assessment was undertaken by Karna et al. [18••], Sowers et al. [19••], and Kastury et al. [65••] during the assessment of plumbojarosite treatment efficacy. Similar to RBA results, IVBA assessment showed dramatic reductions in Pb IVBA resulting in TERs of 0.0001–0.23 (using USEPA Method 1340). Similarly, when As IVBA was assessed after plumbojarosite treatment, Kastury et al. ([65••] reported a TER of 0.38. The high efficiency of conversion of soil Pb species to plumbojarosite, coupled with low Pb RBA/IVBA, provides strong evidence for the immobilization efficacy of this remediation strategy. In addition, the presence of jarosite phases following speciation analysis is a reliable indicator of successful soil immobilization.
Conclusions
Although a range of soil amendments have been used to facilitate Pb/As immobilization in contaminated soil, varying treatment efficacies have been reported as a consequence of differences in amendment and soil physicochemical properties, application rates, contamination concentration/speciation, and operational disparities using in vivo and in vitro assessment techniques. Particularly, the use of different types of in vitro assays may affect treatment efficacy outcomes by virtue of differences in the solution and pH composition, which makes data comparison between studies difficult to interpret. Therefore, standardization of assessment methodologies is essential to overcome the limited ability to compare immobilization efficacy between disparate studies. While expense and ethical considerations limit RBA studies, further effort using in vivo models is needed to validate simple, inexpensive in vitro assays for predicting Pb/As RBA in immobilized soil. Reliable IVBA assays, suited to heterogenous soil systems, are required to minimize the need for RBA assessment and to provide a standardized approach for immobilization comparison. Despite these discrepancies among study designs and reporting of Pb immobilization efficacy, when TER of these different soil amendment strategies are compared, plumbojarosite treatment appears to be the most effective Pb immobilization technique for reducing Pb exposure in situ. However, validation of these initial studies with larger data sets and establishing long-term efficacy, particularly under field conditions are still needed for this newly developed technique.
This review also highlights that further research is needed to gain a mechanistic understanding of processes that facilitate the decreases in Pb/As RBA following soil immobilization. Determination of speciation changes post-immobilization is important as a lines-of-evidence approach for in situ immobilization success; however, additional information is needed to elucidate in vivo processes (e.g., Pb-P formation, Pb co-precipitation, and Pb/Fe competition for DMT-1 across the gastrointestinal tract) that impact Pb/As RBA. Conceivably, in vivo processes could be exploited through nutritional supplements as a complementary risk minimization strategy to soil immobilization. Although limited studies have assessed the impact of soil-amendment aging on Pb/As immobilization, this information is critical to understand treatment longevity, the requirements for supplementary amendment additions, and regulatory acceptance.
Immobilization efficacy is routinely expressed as a reduction in percent Pb/As RBA/IVBA or as a TER. However, the success of an immobilization strategy should be set in the context of soil guidance values and whether post-treatment exposure would result in an acceptable risk when bioavailability reductions are incorporated into exposure assumptions. This simple calculation is often overlooked but is critically important for treatment validation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Henry H, Naujokas MF, Attanayake C, Basta NT, Cheng Z, Hettiarachchi GM, et al. Bioavailability-based in situ remediation to meet future lead (Pb) standards in urban soils and gardens. Environ Sci & Technol. 2015;49:8948–58.
D’amore J, Al-Abed S, Scheckel K, Ryan J. Methods for speciation of metals in soils: a review. J Environ Qual. 2005;34:1707–45.
Kumpiene J, Lagerkvist A, Maurice C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Manag. 2008;28:215–25.
Miretzky P, Fernandez-Cirelli A. Phosphates for Pb immobilization in soils: a review. Environ Chem Letters. 2008;6:121–33.
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, et al. Remediation of heavy metal (loid)s contaminated soils–to mobilize or to immobilize? J Hazard Mater. 2014;266:141–66.
• Palansooriya KN, Shaheen SM, Chen SS, Tsang DC, Hashimoto Y, Hou D, et al. Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int. 2020:134:105046. This review article summarized immobilization efficacy of a variety of soil amendments where complementary, non bioavailability-based methods were used for treatment efficacy assessment.
Scheckel KG, Diamond GL, Burgess MF, Klotzbach JM, Maddaloni M, Miller BW, et al. Amending soils with phosphate as means to mitigate soil lead hazard: a critical review of the state of the science. J Toxicol Environ Health, Part B. 2013;16(6):337–80.
Zeng G, Wan J, Huang D, Hu L, Huang C, Cheng M, et al. Precipitation, adsorption and rhizosphere effect: the mechanisms for phosphate-induced Pb immobilization in soils—a review. J Hazard Mater. 2017;339:354–67.
Basta N, Ryan J, Chaney R. Trace element chemistry in residual-treated soil: key concepts and metal bioavailability. J Environ Qual. 2005;34:49–63.
McLaughlin MJ. Bioavailability of metals to terrestrial plants. In: H.E A, editor. Bioavailability of metals in terrestrial ecosystems: importance of partitioning for bioavailability to invertebrates, microbes, and plants. Pensacola: SETAC; 2002.
Hettiarachchi GM, Pierzynski GM. Soil lead bioavailability and in situ remediation of lead-contaminated soils: a review. Environ Prog. 2004;23:78–93.
Sparks DL. Environmental soil chemistry. 2nd ed. New York, NY, USA: Elsevier; 2003.
Attanayake CP, Hettiarachchi GM, Ma Q, Pierzynski GM, Ransom MD. Lead speciation and in vitro bioaccessibility of compost-amended urban garden soils. J Environ Qual. 2017;46:1215–24.
Beak DG, Basta NT, Scheckel KG, Traina SJ. Bioaccessibility of lead sequestered to corundum and ferrihydrite in a simulated gastrointestinal system. J Environ Qual. 2006;35(6):2075–83.
Miretzky P, Cirelli AF. Remediation of arsenic-contaminated soils by iron amendments: a review. Crit Rev Environ Sci Technol. 2010;40:93–115.
Bradham KD, Diamond GL, Burgess M, Juhasz A, Klotzbach JM, Maddaloni M, et al. In vivo and in vitro methods for evaluating soil arsenic bioavailability: relevant to human health risk assessment. J Toxicol Environ Health, Part B. 2018;21:83–114.
Bradham KD, Diamond GL, Nelson CM, Noerpel M, Scheckel KG, Elek B, et al. Long-term in situ reduction in soil lead bioavailability measured in a mouse model. Environ Sci & Technol. 2018;52:13908–13.
•• Karna RR, Noerpel MR, Nelson C, Elek B, Herbin-Davis K, Diamond G, et al. Bioavailable soil Pb minimized by in situ transformation to plumbojarosite. PNAS 2021:118. https://doi.org/10.1073/pnas.2020315117. This research article is the first report of plumbojarosite formation technique for lead contaminted soil remediation.
•• Sowers TD, Bone SE, Noerpel MR, Blackmon MD, Karna RR, Scheckel KG, et al. Plumbojarosite remediation of soil affects lead speciation and elemental interactions in soil and in mice tissues. Environ Sci & Technol 2021:55:15950–60. This research article demonstrates using XAS techniques that plumbojarosite transitions through gastrointestinal system without being altered.
Sowers TD, Nelson CM, Diamond GL, Blackmon MD, Jerden ML, Kirby AM, et al. High lead bioavailability of indoor dust contaminated with paint lead species. Environ Sci & Technol. 2020;55:402–11.
Bradham KD, Green W, Hayes H, Nelson C, Alava P, Misenheimer J, et al. Estimating relative bioavailability of soil lead in the mouse. J Toxicol Environ Health, Part A. 2016;79:1179–82.
Bradham KD, Laird BD, Rasmussen PE, Schoof RA, Serda SM, Siciliano SD, et al. Assessing the bioavailability and risk from metal-contaminated soils and dusts. Hum Ecol Risk Assess. 2014;20:272–86.
•• Sowers TD, Nelson CM, Blackmon MD, Jerden ML, Kirby AM, Diamond GL, et al. Interconnected soil iron and arsenic speciation effects on arsenic bioaccessibility and bioavailability: a scoping review. J Toxicol Environ Health, Part B. 2022:25:1–22. This review article conducted a meta-analysis of soil As speciation and Fe content to assess their relationship to As IVBA/RBA .
Casteel SW, Weis CP, Henningsen GM, Brattin WJ. Estimation of relative bioavailability of lead in soil and soil-like materials using young swine. Environ Health Pers. 2006;114:1162–71.
Roberts SM, Munson JW, Lowney YW, Ruby MV. Relative oral bioavailability of arsenic from contaminated soils measured in the cynomolgus monkey. Toxicol Sci. 2007;95:281–8.
Roberts SM, Weimar WR, Vinson J, Munson JW, Bergeron RJ. Measurement of arsenic bioavailability in soil using a primate model. Toxicol Sci. 2002;67:303–10.
Duan L, Palanisami T, Liu Y, Dong Z, Mallavarapu M, Kuchel T, et al. Effects of ageing and soil properties on the oral bioavailability of benzo [a] pyrene using a swine model. Environ Int. 2014;70:192–202.
Li H-B, Li M-Y, Zhao D, Li J, Li S-W, Juhasz AL, et al. Oral bioavailability of As, Pb, and Cd in contaminated soils, dust, and foods based on animal bioassays: a review. Environ Sci & Technol. 2019;53:10545–59.
Stevens BN, Betts AR, Miller BW, Scheckel KG, Anderson RH, Bradham KD, et al. Arsenic speciation of contaminated soils/solid wastes and relative oral bioavailability in swine and mice. Soil Syst. 2018;2:27.
Bradham KD, Nelson C, Juhasz AL, Smith E, Scheckel K, Obenour DR, et al. Independent data validation of an in vitro method for the prediction of the relative bioavailability of arsenic in contaminated soils. Environ Sci & Technol. 2015;49:6312–8.
Bradham KD, Scheckel KG, Nelson CM, Seales PE, Lee GE, Hughes MF, et al. Relative bioavailability and bioaccessibility and speciation of arsenic in contaminated soils. Environ Health Pers. 2011;119:1629–34.
Ruby MV. Bioavailability of soil-borne chemicals: abiotic assessment tools. Hum Ecol Risk Assess. 2004;10:647–56.
Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ Sci & Technol. 1996;30:422–30.
Brattin W, Drexler J, Lowney Y, Griffin S, Diamond G, Woodbury L. An in vitro method for estimation of arsenic relative bioavailability in soil. J Toxicol Environ Health, Part A. 2013;76:458–78.
Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, et al. In vitro assessment of arsenic bioaccessibility in contaminated (anthropogenic and geogenic) soils. Chemosphere. 2007;69:69–78.
Juhasz AL, Scheckel KG, Betts AR, Smith E. Predictive capabilities of in vitro assays for estimating Pb relative bioavailability in phosphate amended soils. Environ Sci & Technol. 2016;50:13086–94.
Kastury F, Placitu S, Boland J, Karna RR, Scheckel KG, Smith E, et al. Relationship between Pb relative bioavailability and bioaccessibility in phosphate amended soil: Uncertainty associated with predicting Pb immobilization efficacy using in vitro assays. Environ Int. 2019:131. https://doi.org/10.1016/j.envint.2019.104967.
Tack F, Verloo MG. Chemical speciation and fractionation in soil and sediment heavy metal analysis: a review. Int J Environ Anal Chem. 1995;59:225–38.
Basta N, Gradwohl R. Estimation of Cd, Pb, and Zn bioavailability in smelter-contaminated soils by a sequential extraction procedure. J Soil Contam. 2000;9:149–64.
Zimmerman AJ, Weindorf DC. Heavy metal and trace metal analysis in soil by sequential extraction: a review of procedures. Int J Anal Chem. 2010;2010:1–8.
Okoro HK, Fatoki OS, Adekola FA, Ximba BJ, Snyman RG. A review of sequential extraction procedures for heavy metals speciation in soil and sediments. Sci Reports. 2012;1:118.
Rao C, Sahuquillo A, Lopez SJ. A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water Air Soil Pollut. 2008;189:291–333.
Calmano W, Mangold S, Welter E. An XAFS investigation of the artefacts caused by sequential extraction analyses of Pb-contaminated soils. Fresenius’ J Anal Chem. 2001;371:823–30.
Scheckel KG, Ryan JA. Spectroscopic speciation and quantification of lead in phosphate-amended soils. J Environ Qual. 2004;33:1288–95.
Scheckel KG, Impellitteri CA, Ryan JA, McEvoy T. Assessment of a sequential extraction procedure for perturbed lead-contaminated samples with and without phosphorus amendments. Environ Sci Technol. 2003;37:1892–8.
Gräfe M, Donner E, Collins RN, Lombi E. Speciation of metal (loid)s in environmental samples by X-ray absorption spectroscopy: a critical review. Anal Chimica Acta. 2014;822:1–22.
Juhasz AL, Gancarz D, Herde C, McClure S, Scheckel KG, Smith E. In situ formation of pyromorphite is not required for the reduction of in vivo Pb relative bioavailability in contaminated soils. Environ Sci & Technol. 2014;48:7002–9. https://doi.org/10.1021/es500994u.
Kastury F, Smith E, Doelsch E, Lombi E, Donnelley M, Cmielewski PL, et al. In vitro, in vivo, and spectroscopic assessment of lead exposure reduction via ingestion and inhalation pathways using phosphate and iron amendments. Environ Sci & Technol. 2019;53:10329–41. https://doi.org/10.1021/acs.est.9b02448.
Kastury F, Smith E, Lombi E, Donnelley MW, Cmielewski PL, Parsons DW, et al. Dynamics of lead bioavailability and speciation in indoor dust and x-ray spectroscopic investigation of the link between ingestion and inhalation pathways. Environ Science & Tech. 2019;53:11486–95.
Bradham KD, Nelson CM, Diamond GL, Thayer WC, Scheckel KG, Noerpel M, et al. Dietary lead and phosphate interactions affect oral bioavailability of soil lead in the mouse. Environ Sci & Technol. 2019;53:12556–64.
Mayer M, Basta N, Scheckel KG. Using phosphate amendments to reduce bioaccessible Pb in contaminated soils: a meta-analysis. Frontiers Soil Sci. 2022;2:1–14.
Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC. Intestinal phosphate transport. Adv Chronic Kidney Dis. 2011;18:85–90.
Drexler JW, Brattin WJ. An in vitro procedure for estimation of lead relative bioavailability: with validation. Hum Ecol Risk Assess. 2007;13:383–401. https://doi.org/10.1080/10807030701226350.
Obrycki JF, Basta NT, Scheckel K, Stevens BN, Minca KK. Phosphorus amendment efficacy for in situ remediation of soil lead depends on the bioaccessible method. J Environ Qual. 2016;45:37–44. https://doi.org/10.2134/jeq2015.05.0244.
Karna RR, Noerpel MR, Luxton TP, Scheckel KG. Point of zero charge: role in pyromorphite formation and bioaccessibility of lead and arsenic in phosphate-amended soils. Soil Syst. 2018;2:1–19. https://doi.org/10.3390/soilsystems2020022.
Li S-W, Liu X, Sun H-J, Li M-Y, Zhao D, Luo J, et al. Effect of phosphate amendment on relative bioavailability and bioaccessibility of lead and arsenic in contaminated soils. J Hazard Mater. 2017;339:256–63.
Garau M, Garau G, Diquattro S, Roggero PP, Castaldi P. Mobility, bioaccessibility and toxicity of potentially toxic elements in a contaminated soil treated with municipal solid waste compost. Ecotox Enviro Saf. 2019;186:109766–79. https://doi.org/10.1016/j.ecoenv.2019.109766.
•• Rathnayake S, Schwab AP. In situ stabilization of arsenic and lead in contaminated soil using iron‐rich water treatment residuals. J Environ Qual. 2022:51:425–438. This research articles used sequential amendment of Fe and phosphates to demonstrate immobilization of both As and Pb in soil.
Liao Q, He L, Tu G, Yang Z, Yang W, Tang J, et al. Simultaneous immobilization of Pb, Cd and As in soil by hybrid iron-, sulfate- and phosphate-based bio-nanocomposite: effectiveness, long-term stability and bioavailablity/bioaccessibility evaluation. Chemosphere 2021:266:128960. https://doi.org/10.1016/j.chemosphere.2020.128960.
Mele E, Donner E, Juhasz AL, Brunetti G, Smith E, Betts AR, et al. In situ fixation of metal(loid)s in contaminated soils: a comparison of conventional, opportunistic, and engineered soil amendments. Environ Sci Technol. 2015;49:13501–9. https://doi.org/10.1021/acs.est.5b01356.
Silvetti M, Castaldi P, Holm PE, Deiana S, Lombi E. Leachability, bioaccessibility and plant availability of trace elements in contaminated soils treated with industrial by-products and subjected to oxidative/reductive conditions. Geoderma. 2014;214:204–12.
Nandillon R, Lahwegue O, Miard F, Lebrun M, Gaillard M, Sabatier S, et al. Potential use of biochar, compost and iron grit associated with Trifolium repens to stabilize Pb and As on a multi-contaminated technosol. Ecotox Environ Saf 2019:182:109432. https://doi.org/10.1016/j.ecoenv.2019.109432.
Kunhikrishnan A, Choppala G, Seshadri B, Wijesekara H, Bolan NS, Mbene K, et al. Impact of wastewater derived dissolved organic carbon on reduction, mobility, and bioavailability of As (V) and Cr (VI) in contaminated soils. J Environ Manag. 2017;186:183–91.
Zhang D, Ding A, Li T, Wu X, Liu Y, Naidu R. Immobilization of Cd and Pb in a contaminated acidic soil amended with hydroxyapatite, bentonite, and biochar. J Soil Sed. 2021;21:2262–72. https://doi.org/10.1007/s11368-021-02928-9.
•• Kastury F, Tang W, Herde C, Noerpel MR, Scheckel KG, Juhasz AL. Plumbojarosite formation in contaminated soil to mitigate childhood exposure to lead, arsenic and antimony. J Hazard Mater 2021:418: 11486–11495. https://doi.org/10.1016/j.jhazmat.2021.126312. This research article optimised plumbojarosite formation strategy to reduce water and time needed during soil treatment to reduce Pb/As/Sb exposure.
Alasmary Z, Hettiarachchi GM, Roozeboom KL, Davis LC, Erickson LE, Pidlisnyuk V, et al. Phytostabilization of a contaminated military site using Miscanthus and soil amendments. J Environ Qual. 2021;50:1220–32.
Cai M, McBride MB, Li K, Li Z. Bioaccessibility of As and Pb in orchard and urban soils amended with phosphate. Fe oxide and organic matter Chemosphere. 2017;173:153–9.
Cui H, Yang X, Xu L, Fan Y, Yi Q, Li R, et al. Effects of goethite on the fractions of Cu, Cd, Pb, P and soil enzyme activity with hydroxyapatite in heavy metal-contaminated soil. RSC Adv. 2017;7:45869–77.
Defoe PP, Hettiarachchi GM, Benedict C, Martin S. Safety of gardening on lead-and arsenic-contaminated urban brownfields. J Environ Qual. 2014;43:2064–78.
Gu C, Gates BA, Margenot AJ. Phosphate recycled as struvite immobilizes bioaccessible soil lead while minimizing environmental risk. J Clean Prod. 2020;276:122635–43. https://doi.org/10.1016/j.jclepro.2020.122635.
Janus A, Waterlot C, Heymans S, Deboffe C, Douay F, Pelfrêne A. Do biochars influence the availability and human oral bioaccessibility of Cd, Pb, and Zn in a contaminated slightly alkaline soil? Environ Monit Assess. 2018;190:1–13.
Koralegedara NH, Al-Abed SR, Rodrigo SK, Karna RR, Scheckel KG, Dionysiou DD. Alterations of lead speciation by sulfate from addition of flue gas desulfurization gypsum (FGDG) in two contaminated soils. Sci Total Environ. 2017;575:1522–9. https://doi.org/10.1016/j.scitotenv.2016.10.027.
Kříbek B, Nyambe I, Majer V, Knésl I, Mihaljevič M, Ettler V, et al. Soil contamination near the Kabwe Pb-Zn smelter in Zambia: environmental impacts and remediation measures proposal. J Geochem Explor. 2019;197:159–73.
Li F, Li Z, Mao P, Li Y, Li Y, McBride MB, et al. Heavy metal availability, bioaccessibility, and leachability in contaminated soil: effects of pig manure and earthworms. Environ Sci Pollut Res. 2019;26:20030–9.
Manzano R, Diquattro S, Roggero PP, Pinna MV, Garau G, Castaldi P. Addition of softwood biochar to contaminated soils decreases the mobility, leachability and bioaccesibility of potentially toxic elements. Sci Total Environ 2020:739:139946. https://doi.org/10.1016/j.scitotenv.2020.139946.
Obrycki JF, Scheckel KG, Basta NT. Soil solution interactions may limit Pb remediation using P amendments in an urban soil. Environ Pollut. 2017;220:549–56. https://doi.org/10.1016/j.envpol.2016.10.002.
Paltseva AA, Cheng Z, Egendorf SP, Groffman PM. Remediation of an urban garden with elevated levels of soil contamination. Sci Total Environ 2020:722:137965. https://doi.org/10.1016/j.scitotenv.2020.137965
Plunkett SA, Eckley CS, Luxton TP, Johnson MG. The effects of biochar and redox conditions on soil Pb bioaccessibility to people and waterfowl. Chemosphere 2022:294:133675. https://doi.org/10.1016/j.chemosphere.2022.133675.
Sanderson P, Naidu R, Bolan N. The effect of environmental conditions and soil physicochemistry on phosphate stabilisation of Pb in shooting range soils. J Environ Manag. 2016;170:123–30.
Sanderson P, Naidu R, Bolan N, Lim JE, Ok YS. Chemical stabilisation of lead in shooting range soils with phosphate and magnesium oxide: synchrotron investigation. J Hazard Mater. 2015;299:395–403.
Seshadri B, Bolan NS, Choppala G, Kunhikrishnan A, Sanderson P, Wang H, et al. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere. 2017;184:197–206. https://doi.org/10.1016/j.chemosphere.2017.05.172.
Zagury GJ, Rincon Bello JA, Guney M. Valorization of a treated soil via amendments: fractionation and oral bioaccessibility of Cu, Ni, Pb, and Zn. Environ Monit Assess. 2016;188:222. https://doi.org/10.1007/s10661-016-5223-5.
Acknowledgements
FK was supported by the Australian-American Fulbright Association by the Fulbright Future Postdoctoral Scholarship (funded by the Kinghorn Foundation). The authors acknowledge the support of the University of South Australia, Columbia University, Nanjing University, Bennett Aerospace Inc., U.S. Environmental Protection Agency, Office of Research & Development, Zhejiang University and Kansas State University, Research and Extension. Although EPA contributed to this article, the research presented was not performed by or funded by EPA and was not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in this article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Land Pollution
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kastury, F., Li, H., Karna, R. et al. Opportunities and Challenges Associated with Bioavailability-Based Remediation Strategies for Lead-Contaminated Soil with Arsenic as a Co-Contaminant—A Critical Review. Curr Pollution Rep 9, 213–225 (2023). https://doi.org/10.1007/s40726-023-00252-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-023-00252-z