Abstract
Therapeutic antibodies should not only recognize antigens specifically, but also need to be free from developability issues, such as poor stability. Thus, the mechanistic understanding and characterization of stability are critical determinants for rational antibody design. In this study, we use molecular dynamics simulations to investigate the melting process of 16 antigen binding fragments (Fabs). We describe the Fab dissociation mechanisms, showing a separation in the VH–VL and in the CH1–CL domains. We found that the depths of the minima in the free energy curve, corresponding to the bound states, correlate with the experimentally determined melting temperatures. Additionally, we provide a detailed structural description of the dissociation mechanism and identify key interactions in the CDR loops and in the CH1–CL interface that contribute to stabilization. The dissociation of the VH–VL or CH1–CL domains can be represented by conformational changes in the bend angles between the domains. Our findings elucidate the melting process of antigen binding fragments and highlight critical residues in both the variable and constant domains, which are also strongly germline dependent. Thus, our proposed mechanisms have broad implications in the development and design of new and more stable antigen binding fragments.
Similar content being viewed by others
Introduction
Thanks to their specificity and their ability to target diverse molecules, monoclonal antibodies (mAbs) have emerged as an important class of biopharmaceuticals [1,2,3]. However, many antibodies exhibit several issues like self-association, inadequate pharmacokinetics, immunogenic response that may raise production costs and limit their functionality [3,4,5]. In particular, antibodies’ instability may cause important clinical consequences, like increased aggregation propensity, fast clearance or immunogenic response [6,7,8].
In the process of antibody design, mutations are accumulated in the binding fragment to optimize the antibodies and improve their affinity. However, this may result in an unwanted destabilization of the structure [9,10,11]. Additional modifications are thus required to guarantee thermodynamic stability [12]. Therefore, antibodies’ structure needs to be characterized in detail, in order to increase their stability and to help molecular engineering in the production of suitable therapeutics.
The ability of antibodies to specifically recognize their antigens is determined by the antigen binding fragment (Fab). The Fab consists of a heavy and a light chain (LC) and it can be subdivided in a variable (Fv) and a constant region (CH1–CL). The antigen-binding site is located at the top of the Fv region, and it consists of six loops, known as the complementarity determining regions (CDRs). Thanks to their hypervariable sequences and to the numerous conformations that they can adopt in solution, the CDRs give the antibodies the ability to bind a wide repertoire of antigens [13, 14]. Many of the affinity-enhancing mutations happen in the variable VH–VL interface [4]. Changes in this area improve the complementarity of the binding site towards the antigen, modifying the loop conformations or rotating the VH–VL orientation [15,16,17]. Mutations in the paratope to achieve higher affinity are reflected in a consequent loss of flexibility of the loops [10, 16, 18]. Specific point mutations in the CDR-H3 and CDR-L3 loops also contribute to achieve a high bispecific IgG yield and avoid HC–LC mispairing [19, 20]. The loop region not only determines the ideal folding for the binding, but it contributes, together with the protein core, to stabilize the structure [21]. Therefore, optimization of both the CDR region and the framework can substantially enhance biophysical properties like stability [22, 23].
The two Fab constant domains, CH1–CL, play an important role in stabilizing the Fab and in the binding process. In fact, not only a mutual stabilization occurs across the VH–VL and CH1–CL interfaces, but also the CH1–CL domains can improve the antigen–antibody complementarity [24, 25]. Engineering the interface of antibodies is currently a well-established technique to obtain novel and better performing therapeutics, as bispecific antibodies [26, 27]. The first engineering attempts led to a successful heterodimerization of the two heavy chains (HCs), by modifying few residues in the third constant domain, the CH3–CH3, using the Knob-into-Hole (KiH) technology or charge inversions [28,29,30]. More recently, the introduction of KiH mutations and charge inversions in the Fab interfaces or applying the domain crossover addressed the challenge of the correct LC–HC pairing, to obtain the right antigen binding fragment [31]. Consequently, the study of the CH1–CL interface is highly relevant to help the development of novel antibodies formats.
We studied the mechanism of dissociation for a dataset composed by 16 crystal structures in which four HCs were paired with four κ LCs [32], characterizing the key determinants for antibody stability. We reveal the role of the CDR loops in the dissociation process and describe possible mechanisms of dissociation in the Fv and in the CH1–CL, pointing out the contacts that are relevant to keep the domains together. The detailed analyses of the instability of the Fab structures and the mechanism of the consequent domains’ dissociation provide an important understanding for modifications and improvements in engineering therapeutic antibodies.
Methods
Dataset
Four HC germlines, IGHV1-69 (H1-69), IGHV3-23 (H3-23), IGHV5-51 (H5-51) and IGHV3-53 (H5-53) and four LC germlines (all κ), IGKV1-39 (L1-39), IGKV3-11 (L3-11), IGKV3-20 (L3-20) and IGKV4-1 (L4-1) have been previously combined and crystalized by Teplyakov et al. [32]. These genes have been selected because they are frequently used [33]. The resultant 16 Fab structures differ in their sequences and structures, except for the CDR-H3 loop sequence that is grafted identically to the four HC germlines. The dataset is provided by Teplyakov et al. with experimental information, e.g., crystal structures and melting temperatures [32].
Structure preparation
Crystal structures of the 16 antigen binding fragments are available in the Protein Data Bank (PDB) [34] with the PDB codes 5I15, 5I16, 5I17, 5I18, 5I19, 5I1A, 5I1C, 5I1D, 5I1E, 5I1G, 5I1H, 5I1I, 5I1J, 5I1K, 5I1L, 4KMT. Experimental structural information was available for all considered systems. Figure 1a shows the 16 possible HC–LC pairings and the melting temperatures of the resultant Fab structures. The starting structures for simulations were prepared in MOE (Molecular Operating Environment, Chemical Computing Group, version 2018.01) using the Protonate3D tool [35, 36]. We added the missing side chains and modelled the missing loops. We did not include the cysteines forming a disulfide bond at the C-terminal end of the Fab structures, to avoid an additional stabilization of the CH1–CL domains. The annotation of the CDR loops follows the Kabat numbering scheme [37]. The germlines differ in loop lengths, therefore the numeration of the same loop in different germlines can differ.
Molecular dynamics simulations
We solvated the crystal structures in cubic water boxes of TIP3P water molecules with a minimum wall distance of 10 Å to the protein, using tleap tool of the AmberTools19 package [38,39,40]. Parameters of AMBER force field 14SB were used [41]. Each system was equilibrated using a multistep equilibration protocol [42].
After the equilibration, we performed 1 µs of conventional Molecular Dynamics (cMD) simulations in the NpT ensemble to characterize the bound state of the Fabs. The simulations were performed using CUDA implementation of the particle mesh Ewald method [43]. All bond lengths involving hydrogen atoms were restrained using the SHAKE algorithm, with a time step of 2.0 fs [44]. We used the Berendsen algorithm to set the system to atmospheric pressure (1 bar) [45] and the Langevin thermostat to maintain the temperature of 300 K during the simulations [46].
Umbrella sampling simulations
The crystal structures were solvated in cubic water boxes of TIP3P water molecules with a minimum wall distance of 25 Å to the protein and then equilibrated, using the setup described above. A bigger box was used to ensure that the protein does not interact with its periodic images during the dissociation of the domains. To induce the dissociation between the domains, Umbrella Sampling (US) [47] simulations were performed in GROMACS [48,49,50,51]. As collective variable (CV), we chose the distance between the center of mass (COM) of the LC and HC. We performed pulling simulations on the equilibrated structures, to generate starting structures for US simulations [52]. The pulling took place along the y and z axis over 500 ps, using a spring constant of 1000 kJ mol−1 nm−2 and a pull rate of 0.005 nm ps−1. An asymmetric distribution of sampling windows was used: the umbrella windows extended between a COM distance of 1.7 and 4 nm with a step size of 0.1 nm between 1.7 and 2 nm, a step size of 0.05 nm between 2 and 2.5 nm, and again a step size of 0.1 nm between 2.5 and 4 nm. Such spacing allowed a more detailed sampling of the region in which the dissociation between the domains happens. After a brief NPT equilibration, each window was run for 50 ns at a constant temperature of 310 K, using the same spring constant as in the pulling simulation. The first 5 ns of each umbrella simulation were excluded from the analysis to avoid conformational states that are not fully equilibrated. The convergence of the US runs is assessed via trajectory splitting (SI Fig. 1a).
The multistate Bennett acceptance ratio estimator (MBAR) implemented in pyMBAR has been used to reweight the US trajectories [53]. This allows the estimation of the potential of mean force (PMF) and a direct way to calculate errors. The reweighted PMF curves for each system are plotted in SI Fig. 1b.
Finally, we concatenated the single US runs, in order of increasing CV, obtaining one single simulation for each system.
Transition from bound state to encounter complex
We identified three descriptors that fully characterize the undissociated state of the system: the fraction of native contacts (q) with respect to the starting structure and the distance between the COM of the two variable domains (d1) and of the two constant domains (d2). Therefore, these descriptors were calculated for the cMD simulations, to describe the bound state, and US trajectories of each system using cpptraj [39]. The descriptors q, d1 and d2 show a bell-shaped distribution during the cMDs. To be able to describe the dissociation process, we defined a state function, which is used to classify any structure in our simulation based on the above-mentioned descriptors. To this end, we fitted a decreasing sigmoidal function on the right side of the bell-shaped distribution of d1 and d2, since the distance between the domains increases while the domains separate. An increasing one, instead, can be fitted on the left side of the bell-shaped distribution of q, since the fraction of native contacts decreases during dissociation (SI Fig. 2a). The parameters (slope and turning point) obtained by the fitting procedure are then used to project the sigmoidal functions on the values of d1, d2 and q sampled during the US simulations (SI Fig. 2b). To this end, we used a logistic function:
where s represents the slope of the curve and x0 the turning point.
At this point, we have three logistic functions, covering a [0, 1] y-range, over the range of values sampled in the US for each descriptor. The multiplication of the three sigmoids results in the state function. State 1 corresponds to the bound state, and it smoothly approaches 0, when the dissociation starts. We call the state corresponding to 0 ‘encounter complex’, as it is not bound anymore but not yet completely unbound. The state definition can be applied to identify in each US simulation the point in time in which the whole Fab structure transits from a bound state to an encounter complex (SI Fig. 2c).
Transition from encounter complex to unbound state
We used the fraction of native contacts and SASA to define the transition from the encounter complex to the unbound state. These descriptors can properly describe the process of interest, and in addition, they reach a plateau when the two domains are completely separated. A sigmoidal function can be fitted on the timeseries of the two descriptors during the US simulations (SI Fig. 3a). The two curves are then normalized and multiplied. This results in a state function, where 1 represents the structure in the bound state and in the process of dissociation and 0 corresponds to the completely unbound structure (SI Fig. 3b). The time of transition from the encounter complex to the unbound state corresponds to the frame in the trajectory where the curve approaches 0.
Analysis of interface contacts
The interdomain atomic interactions have been computed using the GetContacts software [54]. This software allows to visualize the evolution of the interdomain contacts during the simulation, specifying the type. The so-called flareplots allow a schematic visualization of the development of the contacts over the simulations. An exemplary representation of flareplots is illustrated in SI Fig. 4b. We calculated the overall number of contacts, which include hydrogen bonds (backbone/backbone, side chain/backbone and side chain/side chain), salt bridges, pi-cation, pi-stacking, T-stacking, hydrophobic. Van der Waals interactions were excluded. The contacts are defined using the default geometrical criteria implemented in GetContacts [54]. In order to describe the mechanism of dissociation in the Fv and in the CH1–CL region, we grouped together the residues belonging to the same loops or β-strands. The secondary structure has been assigned using STRIDE [55]. However, in the Fv region, the Kabat numbering scheme to identify the CDR loops has been respected (SI Fig. 4) [37]. The occurrence of the contacts during the US simulations has been reweighted using pyMBAR tool [53].
Interdomain orientation
The mutual orientation between the VH–VL domains is calculated using the ABangle tool [56]. It describes the orientation between the domains using five angles in degree, four bend angles (HC1, HC2, LC1, LC2) and one torsion angle (HL), plus a distance (dc) in Å. Following the same criteria, the OCD tool has been used to calculate the mutual orientations of the CH1–CL domains, represented as well by four bend angles (cHC1, cHC2, cLC1, cLC2) and one torsion angle (cHL), and a distance (cdc) in Å [57].
Results
The main aim of this work is to characterize one of the many determinants of protein thermostability, i.e. the domain–domain dissociation. Protein denaturation caused by high temperatures does not necessarily involve breaking of the covalent bonds in the polypeptide backbone, but rather the hydrogen bonds and non-covalent interactions tend to rearrange, leading to a less ordered state [58]. Also, the quaternary structure can be involved in this process, as dissociation or spatial rearrangement of the proteins’ subunits can occur. Here, we investigate the domain dissociation as one of the first steps of the melting process. To do this, we performed US simulations on 16 Fab structures to simulate their dissociation process. The systems in the dataset are the result of different LC–HC pairings, which are shown in Fig. 1a. Teplyakov et al. previously crystalized them and measured the melting temperature for each system [32]. The structures share the same constant region and the same CDR-H3 loop. The framework of the variable region and the other five CDR loops, instead, differ between the germlines.
The 16 Fabs break apart following two different pathways: the dissociation between the LC and HC can start in the Fv and continue in the CH1–CL region or vice versa (SI Fig. 5). In both regions, the dissociation follows a two-steps mechanism: the bound structure evolves in an intermediate state that we name encounter complex and finally dissociates completely (SI Fig. 6). The identification of these three states is fundamental to characterize the dissociation process.
Correlation between experimental data and computational results
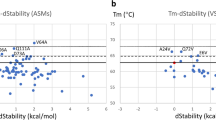
The mechanistic understanding of the melting process in Fab structures is not clear yet. Differential scanning calorimetry (DSC) is one of the most common techniques to experimentally assess protein stability. In our simulation setup, instead of increasing the temperature, we applied a force that dissociates the two domains to provide a potential mechanism of the first steps of the melting process. Here, we make the assumptions that: (a) the domain dissociation simulated via US simulations can partially describe the process that the protein undergoes during melting; (b) the transition from the bound state to the encounter complex is a crucial contributor to thermal stability and thus can be used for its prediction. Therefore, for each system, we estimated the PMF curves resulting from the US simulations until this transition point, and we calculated the depth of the global minimum corresponding to the bound structure. The energy depth correlates with the melting temperatures, with a Pearson coefficient of 0.79 (Fig. 1b).
Correlation of the energy depth with the experimentally determined melting temperatures for 16 antigen binding fragments. a Table including the PDB codes of the 16 systems (dataset from Teplyakov et al. [30]), the heavy and light chain germline type that they comprise, and their experimentally measured melting temperatures. b Correlation between the energy depth of the bound Fab and its melting temperature. Each system is color coded according to a. The errors are represented by grey lines
Structural analysis of the dissociation
After proving the reliability of our computational results, we performed a structural analysis to highlight the residues that stabilize or destabilize the interface. Focusing on the paratope, the CDR-H3 sequence is conserved in all the structures, while the sequences of the other loops differ. Therefore, we compared the interactions of the LC CDR loops with the CDR-H3 loop. We found a high germline dependency when looking at the residues that stabilize the CDR-H3 loop.
Interactions of the CDR-H3 loop with the CDR-L3 loop
Using the GetContacts tool [54], we calculated the occurrence of contacts that are present between the CDR-L3 and the CDR-H3 loop during the US simulations in the bound state and in the encounter complex for each system and reweighted it using pyMBAR [53, 54]. Figure 2a, and Figure 2b show the occurrence of contacts between the two loops respectively in the bound state and in the encounter complex. The residues in the CDR-L3 loop that interact with the CDR-H3 loop are mostly conserved between the four LC germlines, except the third residue of the CDR-L3 loop, which is the Tyr92 in the L3-20 and the Tyr97 in the L4-1 germlines, the Arg91 in the L3-11 germline and the Ser91 in the L1-39. The Arg91 in the L3-11 germline makes the highest number of contacts with several residues in the CDR-H3 loop (Fig. 2c). The CDR-L3/CDR-H3 contacts in the L1-39 germline occur more frequently than in the L3-20 and L4-1 germline. This is mainly because of the Gln89 and the Ser91 which make strong H-bonds with, respectively, the Gly104 and the Tyr103 in the CDR-H3 loop (Fig. 2d). Most of the CDR-L3/CDR-H3 contacts are H-bonds, apart from the Tyr92 (L3-20 germline)-Tyr103 which form a pi-stacking interaction. During dissociation, the occurrence of the contacts between the CDR-H3 and the CDR-L3 loop decreases (Fig. 2b). Some interactions are still present in the 5I16, 5I1A and 5I1G, thanks to the Gln89 and the Arg91 in the CDR-L3 loop of the L3-11 germline, and in the L1-39 germline system, between the Ser91 in the CDR-L3 loop and the Tyr103 in the CDR-H3 loop.
Interactions of the CDR-H3 loop with the CDR-L3 loop. a Occurrence of contacts between the CDR-L3 and the CDR-H3 loop in the bound state of each system. b Occurrence of contacts between the CDR-L3 and the CDR-H3 loop in the encounter complex of each system. c Top view of an exemplary Fv region comprising the L3-11 germline. The CDR-H3 loop is colored in light red, the CDR-L1 loop in light orange, the CDR-L2 in green and the CDR-L3 loop in light blue. The residues involved in loop interactions are illustrated as sticks and the contacts that they make are represented by black lines. Labels are shown for the residues in the CDR-L3 loop. d Top view of an exemplary Fv region comprising the L1-39 germline. The coloring follows the one in d. The residues involved in loop interactions are illustrated as sticks and the contacts that they make are represented by black lines. Labels are shown for the residues in the CDR-L3, CDR-L2 and CDR-L1 loops
Interactions of the CDR-H3 loop with the CDR-L1 loop and the CDR-L2 loop
Also the CDR-L1 and CDR-L2 loops interact with the CDR-H3 loop, but in these cases the contacts are much less frequent compared to the CDR-L3/CDR-H3 loops interactions (up to 100% the occurrence of contacts in the bound state in the latter pair, compared to a maximum of 60% in the formers). The CDR-L1 loop shows low variability in sequence from one germline to the other, with the highest difference in the L4-1 germline, whose CDR-L1 loop is longer than in the other germlines. The same variability is also present in the CDR-L2 loops, however, in this case, the loop length stays constant in all germlines. The contacts between the CDR-H3 and the CDR-L1/CDR-L2 loops have been calculated and reweighted following the same protocol described above for the CDR-L3/CDR-H3 loops. SI Fig. 7a shows the CDR-L1/CDR-H3 contacts in the bound state of the structures. It is evident that the CDR-L1 loop in the germline L1-39 makes the highest number of contacts, in comparison to the other germlines, thanks to the contribution of the Asn34, which makes multiple H-bonds with the Tyr103, Gly104 and Glu105 in the CDR-H3 loop. Another relevant contact is the one between the Tyr32 in the CDR-L1 loop and the Tyr103 in the CDR-H3 loop. This contact is also present in the other LC germlines, but with a much lower occurrence. In the dissociation process, the CDR-L1/CDR-H3 contacts are only present in the system derived from the L1-39 germline, in which the Asn34 still strongly interacts with the CDR-H3 loop (SI Fig. 7b). The CDR-L2 loop, instead, makes less interactions with the CDR-H3 loop (SI Fig. 7c). Quite occurrent is the contact between the Gln55 in the CDR-L2 loop of L1-39 germline and the Asp107 in the CDR-H3 loop. However, this interaction is almost not visible anymore during dissociation (SI Fig. 7d).
CH1–CL interdomain contacts
All the germlines share the same constant region. Figure 3a shows the occurrence of the contacts in the bound state of the systems. Only the contacts with an occurrence greater than 0.3 are shown. The numeration of the constant region is described in SI Fig. 8. Most of the contacts are H-bonds or hydrophobic ones. Only the Asp122 and the Glu123 in the CL make a salt bridge respectively with the Lys220 and the Lys215 in the G strand of the CH1 (Fig. 3c). A lysine is also present in the G strand of the LC, in position 207. However, it does not have any negatively charged partner in the opposite chain to establish a salt bridge. Seldomly, the Lys207 in the LC makes H-bonds with several residues in the AB strand of the HCs. These contacts are not shown in Fig. 3, because their occurrence is low. Leu135 in the LC has also an important role: thanks to its central position, it makes several hydrophobic contacts with strand B (Ala143), D (Phe172) and E (Val187) in the HC. Generally, the CH1–CL interdomain contacts still take place in the encounter complex, even if with a lower occurrence compared to the bound state (Fig. 3b).
CH1–CL interdomain contacts. a Representation of the occurrence of the contacts in the bound state of the simulations. The residues that make contacts are shown in the y axis: the first residue belongs to the CL and the second one to CH1. The capital letters represent the strands or loops where these residues are located. b Representation of the occurrence of the contacts in the encounter complexes. c Stick representation of the paired residues in the CH1–CL interface, with specification of the type of contacts that they make. The light grey domain is the CL, the dark grey is the CH1
Mechanism of dissociation in the Fv
Generally, the US simulations lead to dissociation in both regions (Fv and CH1–CL) at different points in time. Only for two systems (5I1C and 5I1J) the dissociation is only observed in the Fv region (SI Fig. 5). A partial dissociation and a rearrangement of the CH1–CL domains of these two systems is observable in later stages of the pulling simulations, but this event is not visible in the selected windows (maximum distance between the COMs of the LC and HC equal to 4 nm). We represent the interdomain contacts with the so-called flareplots, grouping the residues belonging to the same strand or loop (SI Fig. 4). To describe how the interdomain contacts develop during the dissociation process, we consider snapshots of 2 ns starting from the beginning of the US simulations, and from the bound–encounter transition points. This timeframe can give a good statistic of the contacts that take place at the beginning of the simulation and in the transition state. We neglect the encounter–unbound transition because the unbound state is not relevant for our study. Figure 4 shows an exemplary representation of the interdomain contacts that are present in one system in these timeframes. The result stays generally consistent for all the systems, with few differences observed. Focusing on the Fv region, the bound structure shows multiple contacts between the β-strands h_C, h_D, h_E, h_H, h_I and the neighboring loops in the HC and the β-strands l_C, l_D, l_G and l_H and the neighboring loops in the LC (Fig. 4a). At the transition point from bound to encounter complex, some contacts are already missing. The CDR-H3 loop (h_HI) still makes contacts with the CDR-L1 (l_BC) and the CDR-L3 (l_GH) loops (Fig. 4b). Some β-strands are still involved in interdomain contacts at this point of dissociation, but these contacts can evolve quite fast and can differ from one system to the other, depending on the domain orientation.
Mechanism of dissociation in the Fv region. Exemplary representation of the contacts in the Fv region in the bound state (a), at the bound–encounter transition point (b). On the left side, the contacts are represented by flareplots. On the right side, a structural representation of the state: in the background, the ensemble of conformations that an exemplary structure adopts in the selected timeframes; in front, a cartoon representation of the structure at the first frame of each timespan. The β-strands involved in interdomain contacts are colored according to the flareplots. The light chain (in light grey) is on the right side, the heavy chain (in dark grey) is on the left
Mechanism of dissociation in the CH1–CL region
In order to compare different interfaces, we repeated the same procedure as for the Fv region also for the CH1–CL. We plotted the contacts that exist in the CH1–CL interface, showing flareplots in which the residues that belong to the same loops or β-sheets are grouped together (SI Fig. 4). Also for this region, we looked at the contacts that are present in the CH1–CL interface considering snapshots of 2 ns at the beginning of the US simulations and at the bound–encounter transition point. Figure 5 shows the interdomain contacts in a representative system during dissociation. The findings are transferable also to the other systems with minor differences. The bound state is characterized by contacts that involve the β-strands A, B, D, E and G and the loops AB and DE in both chains (Fig. 5a). When the structure transits from a bound state to the encounter complex state, it can adopt two different conformations: either the h_G strand interacts with the l_AB loop (Fig. 5b), or l_G interacts with h_AB (Fig. 5c). In any case, the G strand of one of the two chains is the first element to lose contacts with the opposite domain. The only two salt bridges that are present in the CH1–CL structure are formed between h_G and l_AB (Fig. 3). In the dissociation pathway shown in Fig. 5c, these salt bridges are missing and the whole structure is kept together only by hydrophobic interactions and H-bonds.
Mechanism of dissociation in the CH1–CL region. Representation of the contacts in the CH1–CL region in the bound state (a) and at the transition point from the bound state to the encounter complex (b, c). A structural depiction of the bound state is also provided: in the background, the representation of the ensemble of structures; in front, a cartoon representation. The β-strands involved in interdomain contacts are colored according to the flareplots. The light chain (CL, in light grey) is on the right side, the heavy chain (CH1, in dark grey) is on the left. The bound–encounter transition can happen following two mechanisms depicted in b and c
Shifts in the interdomain orientation during dissociation
We analyzed how the angles that describe the movement in the interface change during the dissociation process. SI Fig. 9a shows the shifts in the VH–VL interface angles for each system until the end point of dissociation, calculated with the ABangle tool [56]. The standard deviation of each angle during this time has also been calculated and it is shown in each plot. In four systems (5I17, 5I1A, 5I1H and 5I1K) the biggest shift is present in the HL angle. For all the other systems, instead, the bend angles are the ones that change the most during dissociation. The same analysis has been done also for the CH1–CL region, using the OCD tool [57] (SI Fig. 9b). Excluding the 5I1C and the 5I1J that don’t dissociate in the CH1–CL region during our simulations, only three systems show a bigger shift in the torsion angle (5I15, 5I19, 5I1K). In most of the cases, instead, the dissociation causes a change of the bend angles.
Discussion
In this work we structurally characterize the mechanism of dissociation of 16 Fab fragments [32]. This dataset has been chosen because of the availability of experimental data, such as crystal structures and melting temperatures. The melting temperatures have been measured using DSC, one of the most common and efficient techniques to assess protein melting and unfolding, which provides the difference in temperature between a reference and a sample cell [59]. DSC remains unparalleled to assess the thermodynamic stability of proteins [60] and this type of measurement is particularly relevant for antibodies because stability directly impacts their structure and ultimately their function [61, 62]. Thermal unfolding involves loss of tertiary structure, followed by loss of some elements of secondary structure, and general unfolding of the protein [63]. The profile of the temperature-induced unfolding for a whole IgG1 mAb consists of two peaks: the high-temperature peak comprises an overlay of two transitions—reversible unfolding of CH2–CH2 domains followed by irreversible unfolding of the Fab-, instead the low-temperature one displays the melting of the CH3–CH3 domains [64,65,66,67]. Even if the melting process of the whole antibody molecule has been thoroughly studied, the mechanism of unfolding of the domain complexes at the interface level is still unknown. The interface between the domains is stabilized by non-covalent interactions, that are highly susceptible to stress at high temperatures. Therefore, the quaternary structure of the protein will potentially be the first one to be affected in the melting process, resulting in domain dissociation or spatial rearrangement. In antibody design, this is particularly relevant because chain mispairings or non-favorable orientations of the domains can strongly modify the antigen binding site, resulting in a non-functional antibody [68]. For this reason, several studies have been carried out to mutate the interface residues and, on one side, ensure the correct chain pairing, but also increase the overall protein stability [4, 19]. It is therefore important to characterize the interactions that take place in the antibodies’ interface, to avoid functionality or developability issues [69].
In order to address this open question and simulate the domain dissociation, we ran US simulations, which overtake the sampling limits of MD simulations. This enhanced sampling technique is established to study protein–protein binding processes or to assess protein stability [52, 70]. Another advantage of US is the possibility to obtain a reweighted free energy curve that describes the process of interest.
In our simulation, we observe that the bound structure evolves to an encounter complex state, before completely dissociating (SI Figs. 5, 6). However, while the bound state and the encounter complex are well defined, the unbound state shows the highest diversity and thus uncertainty. Therefore, we assume that the melting process represents the transition between the bound state and the encounter complex. The depths of the minima of the free energy curves, that correspond to the energy of the bound state, are compared to the experimentally measured melting temperatures, to assess the reliability of our computational results. The correlation shows that, even if we are neglecting other important aspects connected to thermal stability, the domain dissociation process captured by our simulations represents a critical step in the melting process (Fig. 1b). The analysis of the free energy of the bound states can properly separate the stable from the unstable Fabs. However, systems with the same melting temperature can result in different values of energy associated to the bound state and some outliers can be identified. This is mainly due to the simplification of the highly complex melting process to the domains’ dissociation. Moreover, the dissociation happens in both Fv and CH1–CL regions for 14 systems out of 16. In the Fabs with PDB codes 5I1C and 5I1J, instead, only a full dissociation in the Fv region is visible. This is because of the cutoff that we chose before starting US simulations (4 nm COM distance between LC and HCs, see Methods). For these two systems, in fact, the CH1–CL dissociation starts only at a later stage in the pulling simulations.
Antibodies’ biophysical properties are highly influenced by the germlines and their respective HC and LC pairings [71]. The overall stability depends on the intrinsic structural stability of each domain, as well as on the extrinsic stabilization provided by their interaction [72]. Our 16 Fab structures are the result of different pairings of four HC germlines with four LC germlines (all κ) and, therefore, we were able to derive how different germline pairings influence the Fab stability.
The Fv region, and especially the paratope, often causes instability because of the high variability in sequences and conformations. On the other hand, the loop region couples with the protein core and substantially contributes to the stability of the fold [21]. The germlines in our dataset differ in CDR loops length and sequence, except for the CDR-H3 loop which is conserved. Even if all the loops contribute to shape the paratope, the CDR-H3 loop raises the main interest because it makes on average the highest number of contacts with the antigen [73,74,75]. The contacts between the VL-CDR loops and the CDR-H3 loop provide a meaningful stabilization of the variable region. In particular, the CDR-L3 and CDR-H3 loops lie together in the center of the antigen binding site and are relevant to shape the paratope [16]. Besides, they have comparable diversity in sequence, length and structure [76,77,78]. We compared the effect of CDR loops derived from the different germlines on the same CDR-H3 loop. Our findings suggest that the distinct LC–HC pairings highly influence the stability of the Fab structures. Comparing the CDR loops interactions in the different systems, it is evident that the LC germlines present in the most stable Fabs (L3-11 and L1-39) are also the ones in which the paratope is highly stabilized. Focusing on the CDR-H3/CDR-L3 interactions, when an Arg is present as third residue of the CDR-L3 loop, as in the germline L3-11, it forms more interactions with the CDR-H3 loop rather than when a Tyr or a Ser is in the same position (Fig. 2a). As previously discussed in literature, Arg located in the CDR loops, is a critical determinant for affinity [79,80,81]. In this case, it seems that this residue may play an important role in the stabilization of the CDR-H3 loop, too.
The CDR-L1 and the CDR-L2 loops also interact with the CDR-H3 loop, even if their contacts are less occurrent than the ones between CDR-L3 and CDR-H3 loop (SI Fig. 7). In our dataset, CDR-L1 loops show quite some differences in sequence and length, whereas CDR-L2 loops share the same length but differ in sequence. Loop length variation is proven to be a valid strategy for affinity maturation of antibodies, since it influences tertiary structure and activity simultaneously [82, 83]. However, an enhancement in affinity does not always go hand in hand with a higher stability. In our dataset, for example, the two germlines with longer CDR-L1 loops, L3-20 and L4-1 (respectively 12 and 17 AA) show only few stabilizing contacts with the CDR-H3 loop. Instead, the L1-39 germline, which is also the most stable one, makes the highest number of interactions (SI Fig. 7a). The interactions between CDR-L1 and CDR-H3 loops can mainly be attributed to the Asn34, i.e., the last residue of the CDR-L1 loop in the L1-39 germline, whose contacts with the CDR-H3 loop are still present in the encounter complex (SI Fig. 7b). The presence of the Asn in the variable region can lead to a degradation mechanism, i.e., deamidation, especially when it adopts particular geometries on the tip of a loop [84]. The Asn34 in this case, though, can be highly stabilized by the neighboring Trp35 present in the framework [85].
The CDR loops can adopt several conformations in solution that result in different interactions [75, 86] and this is also confirmed in this work. In fact, the contacts that the CDR loops make in the encounter complexes are not always the same as in the bound state, suggesting a conformational rearrangement of the loops. This is evident, for example, in the CDR-L3/CDR-H3 interactions in the 5I1I, 5I1A and 5I1E (Fig. 2b), where different loops interactions are present in the encounter complex compared to the bound state. Even if a rigid interface is usually associated to a specificity in the binding [10, 87, 88], backbone flexibility allows conformational adjustments that can lead to stabilizing, low-energy interactions [89].
Interdomain contacts are key determinants for stability. Therefore, we studied the constant domain interface, showing that it is mainly characterized by hydrophobic interactions and H-bonds [69]. The β-strands A, B, D, E and the loops AB and DE in both chains are mainly responsible for the interdomain interactions, since they are in the center of the interface (Fig. 3). The only two salt bridges in the CH1–CL interface are formed between two lysines (Lys215, Lys220) in the β-strand G in the HC and the AB loop in the LC. Interestingly, the β-strand G is the first strand to lose its contacts with the partner domain during dissociation (Fig. 5). The encounter complex state, in which the protein–protein interface is partially solvated and contains non-optimal sidechain orientations, is characterized mainly by hydrophobic interactions [90]. Not only the CH1–CL region provides a further stabilization of the Fab fragment [24], but also the latest development in antibody engineering introduced modifications in the CH1–CL interface, obtaining stabilized heterodimeric interfaces, to facilitate the production of bispecific antibodies [31, 68, 91, 92]. Therefore, the study of the interactions in the first constant domain interface is highly relevant for the design of novel therapeutics.
Furthermore, we found exemplary mechanisms of dissociation in the Fv and in the CH1–CL region. In our simulations, we do not sample the unfolding of the single domains, which happens only after the loss of contacts at the interface between the VH–VL and CH1–CL domains [93]. The Fab structures that compose our dataset dissociate in the Fv region and in the CH1–CL at different times (SI Fig. 5), showing high cooperation between the two interfaces [94]. The analysis of the dissociation process in the Fv region shows that several contacts are present between the two domains in the bound state, but especially the interactions between the CDR-H3 and the CDR-L3 loop keep the structures together until complete dissociation (Fig. 4). The dissociation in the CH1–CL region, instead, is characterized by a reorientation of the two domains that leads to a loss of the contacts of the G strand (Fig. 5b, c).
A key aspect in antibody modelling and engineering is the domain orientation, since it highly influences the binding mechanism and the overall affinity [56]. The dissociation affects, of course, the orientation between the LC and the HC. The separation of the two domains can be mainly represented by big shifts in the bend angles, in both the Fv and the CH1–CL region, whereas only few systems show major changes in the HL angle (SI Fig. 9).
Conclusion
Low thermal stability is one of the main issues in rational antibody design. We studied the stability of 16 Fab structures, characterizing their mechanism of dissociation. The paratope stabilization and the interdomain contacts are key determinants for the Fab stability. Our results show that the contacts between the CDR loops are highly germline dependent and especially the ones between the CDR-H3 and the CDR-L3 loop occur during the whole dissociation process, stabilizing the paratope. On the other hand, the Fv and the CH1–CL interface show co-operativity in the stabilization of the Fab. The hydrophobic interdomain contacts and the H-bonds located in the core of the constant domain interface occur during the whole dissociation process, additionally stabilizing the Fab.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Kaplon H, Reichert JM (2019) Antibodies to watch in 2019. MAbs 11:219–238. https://doi.org/10.1080/19420862.2018.1556465
Kaplon H, Muralidharan M, Schneider Z, Reichert JM (2020) Antibodies to watch in 2020. MAbs 12:1703531. https://doi.org/10.1080/19420862.2019.1703531
Chames P, Van Regenmortel M, Weiss E, Baty D (2009) Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol 157:220–233. https://doi.org/10.1111/j.1476-5381.2009.00190.x
Warszawski S, Katz AB, Lipsh R et al (2019) Optimizing antibody affinity and stability by the automated design of the variable light–heavy chain interfaces. PLoS Comput Biol 15:e1007207. https://doi.org/10.1371/journal.pcbi.1007207
Raybould MIJ, Marks C, Krawczyk K et al (2019) Five computational developability guidelines for therapeutic antibody profiling. Proc Natl Acad Sci USA 116:4025–4030. https://doi.org/10.1073/pnas.1810576116
Le Basle Y, Chennell P, Tokhadze N et al (2020) Physicochemical stability of monoclonal antibodies: a review. J Pharm Sci 109:169–190. https://doi.org/10.1016/j.xphs.2019.08.009
Yang X, Xu W, Dukleska S et al (2013) Developability studies before initiation of process development. MAbs 5:787–794. https://doi.org/10.4161/mabs.25269
Cromwell MEM, Hilario E, Jacobson F (2006) Protein aggregation and bioprocessing. AAPS J 8:E572–E579. https://doi.org/10.1208/aapsj080366
Acierno JP, Braden BC, Klinke S et al (2007) Affinity maturation increases the stability and plasticity of the Fv domain of anti-protein antibodies. J Mol Biol 374:130–146. https://doi.org/10.1016/j.jmb.2007.09.005
Fernández-Quintero ML, Loeffler JR, Bacher LM et al (2020) Local and global rigidification upon antibody affinity maturation. Front Mol Biosci 7:182. https://doi.org/10.3389/fmolb.2020.00182
Cauerhff A, Goldbaum FA, Braden BC (2004) Structural mechanism for affinity maturation of an anti-lysozyme antibody. Proc Natl Acad Sci USA 101:3539–3544. https://doi.org/10.1073/pnas.0400060101
Julian MC, Li L, Garde S et al (2017) Efficient affinity maturation of antibody variable domains requires co-selection of compensatory mutations to maintain thermodynamic stability. Sci Rep 7:45259. https://doi.org/10.1038/srep45259
Davies DR, Chacko S (1993) Antibody structure. Acc Chem Res 26:421–427. https://doi.org/10.1021/ar00032a005
Nowak J, Baker T, Georges G et al (2016) Length-independent structural similarities enrich the antibody CDR canonical class model. MAbs 8:751–760. https://doi.org/10.1080/19420862.2016.1158370
Stanfield RL, Takimoto-Kamimura M, Rini JM et al (1993) (1993) Major antigen-induced domain rearrangements in an antibody. Structure (Lond Engl) 1:83–93. https://doi.org/10.1016/0969-2126(93)90024-b
Fernández-Quintero ML, Pomarici ND, Math BA et al (2020) Antibodies exhibit multiple paratope states influencing VH–VL domain orientations. Commun Biol 3:1–14. https://doi.org/10.1038/s42003-020-01319-z
Banfield MJ, King DJ, Mountain A, Brady RL (1997) VL:VH domain rotations in engineered antibodies: crystal structures of the Fab fragments from two murine antitumor antibodies and their engineered human constructs. Proteins Struct Funct Bioinform 29:161–171. https://doi.org/10.1002/(SICI)1097-0134(199710)29:2%3c161::AID-PROT4%3e3.0.CO;2-G
Kuroda D, Tsumoto K (2018) Antibody affinity maturation by computational design. Methods Mol Biol (Clifton NJ) 1827:15–34. https://doi.org/10.1007/978-1-4939-8648-4_2
Joshi KK, Phung W, Han G et al (2019) Elucidating heavy/light chain pairing preferences to facilitate the assembly of bispecific IgG in single cells. MAbs 11:1254–1265. https://doi.org/10.1080/19420862.2019.1640549
Fernández-Quintero ML, Kroell KB, Grunewald LJ et al (2022) CDR loop interactions can determine heavy and light chain pairing preferences in bispecific antibodies. MAbs 14:2024118. https://doi.org/10.1080/19420862.2021.2024118
Billings KS, Best RB, Rutherford TJ, Clarke J (2008) Crosstalk between the protein surface and hydrophobic core in a core-swapped fibronectin type III domain. J Mol Biol 375:560–571. https://doi.org/10.1016/j.jmb.2007.10.056
Kuroda D, Tsumoto K (2020) Engineering stability, viscosity, and immunogenicity of antibodies by computational design. J Pharm Sci 109:1631–1651. https://doi.org/10.1016/j.xphs.2020.01.011
Chang H-J, Jian J-W, Hsu H-J et al (2014) Loop-sequence features and stability determinants in antibody variable domains by high-throughput experiments. Structure 22:9–21. https://doi.org/10.1016/j.str.2013.10.005
Röthlisberger D, Honegger A, Plückthun A (2005) Domain interactions in the Fab fragment: a comparative evaluation of the single-chain Fv and Fab format engineered with variable domains of different stability. J Mol Biol 347:773–789. https://doi.org/10.1016/j.jmb.2005.01.053
Adachi M, Kurihara Y, Nojima H et al (2003) Interaction between the antigen and antibody is controlled by the constant domains: normal mode dynamics of the HEL–HyHEL-10 complex. Protein Sci Publ Protein Soc 12:2125–2131
Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI (2019) Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov 18:585–608. https://doi.org/10.1038/s41573-019-0028-1
Ma J, Mo Y, Tang M et al (2021) Bispecific antibodies: from research to clinical application. Front Immunol 12:1555. https://doi.org/10.3389/fimmu.2021.626616
Ridgway JB, Presta LG, Carter P (1996) “Knobs-into-holes” engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 9:617–621. https://doi.org/10.1093/protein/9.7.617
De Nardis C, Hendriks LJA, Poirier E et al (2017) A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G1. J Biol Chem 292:14706–14717. https://doi.org/10.1074/jbc.M117.793497
Leaver-Fay A, Froning KJ, Atwell S et al (1993) (2016) Computationally designed bispecific antibodies using negative state repertoires. Structure (Lond Engl) 24:641–651. https://doi.org/10.1016/j.str.2016.02.013
Bönisch M, Sellmann C, Maresch D et al (2017) Novel CH1:CL interfaces that enhance correct light chain pairing in heterodimeric bispecific antibodies. Protein Eng Des Sel 30:685–696. https://doi.org/10.1093/protein/gzx044
Teplyakov A, Obmolova G, Malia TJ et al (2016) Structural diversity in a human antibody germline library. MAbs 8:1045–1063. https://doi.org/10.1080/19420862.2016.1190060
de Wildt RM, Hoet RM, van Venrooij WJ et al (1999) Analysis of heavy and light chain pairings indicates that receptor editing shapes the human antibody repertoire. J Mol Biol 285:895–901. https://doi.org/10.1006/jmbi.1998.2396
Berman HM, Westbrook J, Feng Z et al (2000) The Protein Data Bank. Nucleic Acids Res 28:235–242. https://doi.org/10.1093/nar/28.1.235
Molecular Operating Environment (MOE) | MOEsaic | PSILO. https://www.chemcomp.com/Products.htm. Accessed 3 Nov 2020
Labute P (2009) Protonate3D: assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins 75:187–205. https://doi.org/10.1002/prot.22234
Kabat EA, Bilofsky H, Wu TT (1979) Sequences of immunoglobulin chains: tabulation and analysis of amino acid sequences of precursors, V-regions, C-regions, J-chain and [beta] 2-microglobulins. U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health
AmberTools 19—molecular dynamics simulation—My Biosoftware—bioinformatics softwares blog. http://www.mybiosoftware.com/ambertools-1-4-molecular-dynamics-simulation.html. Accessed 11 Dec 2020
Roe DR, Cheatham TE (2013) PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J Chem Theory Comput 9:3084–3095. https://doi.org/10.1021/ct400341p
Jorgensen WL, Chandrasekhar J, Madura JD et al (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935. https://doi.org/10.1063/1.445869
Maier JA, Martinez C, Kasavajhala K et al (2015) ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11:3696–3713. https://doi.org/10.1021/acs.jctc.5b00255
Wallnoefer HG, Liedl KR, Fox T (2011) A challenging system: free energy prediction for factor Xa. J Comput Chem 32:1743–1752. https://doi.org/10.1002/jcc.21758
Salomon-Ferrer R, Götz AW, Poole D et al (2013) Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J Chem Theory Comput 9:3878–3888. https://doi.org/10.1021/ct400314y
Miyamoto S, Kollman PA (1992) Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem 13:952–962. https://doi.org/10.1002/jcc.540130805
Berendsen HJC, Postma JPM, van Gunsteren WF et al (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690. https://doi.org/10.1063/1.448118
Adelman SA, Doll JD (1976) Generalized Langevin equation approach for atom/solid-surface scattering: general formulation for classical scattering off harmonic solids. J Chem Phys 64:2375–2388. https://doi.org/10.1063/1.432526
Torrie GM, Valleau JP (1977) Nonphysical sampling distributions in Monte Carlo free-energy estimation: umbrella sampling. J Comput Phys 23:187–199. https://doi.org/10.1016/0021-9991(77)90121-8
Abraham MJ, Murtola T, Schulz R et al (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Páll S, Abraham MJ, Kutzner C et al (2015) Tackling Exascale software challenges in molecular dynamics simulations with GROMACS. In: Markidis S, Laure E (eds) Solving software challenges for Exascale. Springer, Cham, pp 3–27
Pronk S, Páll S, Schulz R et al (2013) GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics (Oxf Engl) 29:845–854. https://doi.org/10.1093/bioinformatics/btt055
Lindahl A, van der Hess S (2019) GROMACS 2019.2 Source code
Lemkul JA, Bevan DR (2010) Assessing the stability of Alzheimer’s amyloid protofibrils using molecular dynamics. J Phys Chem B 114:1652–1660. https://doi.org/10.1021/jp9110794
Shirts MR, Chodera JD (2008) Statistically optimal analysis of samples from multiple equilibrium states. J Chem Phys 129:124105. https://doi.org/10.1063/1.2978177
GetContacts. https://getcontacts.github.io/. Accessed 3 Nov 2020
Frishman D, Argos P (1995) Knowledge-based protein secondary structure assignment. Proteins 23:566–579. https://doi.org/10.1002/prot.340230412
Dunbar J, Fuchs A, Shi J, Deane CM (2013) ABangle: characterising the VH–VL orientation in antibodies. Protein Eng Des Sel 26:611–620. https://doi.org/10.1093/protein/gzt020
Hoerschinger VJ, Fernández-Quintero ML, Waibl F et al (2021) OCD.py—characterizing immunoglobulin inter-domain orientations. bioRxiv. https://doi.org/10.1101/2021.03.15.435379
Bischof JC, He X (2006) Thermal stability of proteins. Ann N Y Acad Sci 1066:12–33. https://doi.org/10.1196/annals.1363.003
He F, Hogan S, Latypov RF et al (2010) High throughput thermostability screening of monoclonal antibody formulations. J Pharm Sci 99:1707–1720. https://doi.org/10.1002/jps.21955
Gokarn Y, Agarwal S, Arthur K et al (2015) Biophysical techniques for characterizing the higher order structure and interactions of monoclonal antibodies. In: Schiel JE, Davis DD, Borisov OV (eds) State-of-the-art and emerging technologies for therapeutic monoclonal antibody characterization. Biopharmaceutical characterization: the NIST mAb Case Study, vol 2. American Chemical Society, Washington, DC
Wang W, Singh S, Zeng DL et al (2007) Antibody structure, instability, and formulation. J Pharm Sci 96:1–26. https://doi.org/10.1002/jps.20727
Wu S-J, Luo J, O’Neil KT et al (2010) Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel 23:643–651. https://doi.org/10.1093/protein/gzq037
Pollegioni L, Iametti S, Fessas D et al (2003) Contribution of the dimeric state to the thermal stability of the flavoprotein d-amino acid oxidase. Protein Sci Publ Protein Soc 12:1018–1029
Zav’yalov VP, Tishchenko VM (1991) Mechanisms of generation of antibody diversity as a cause for natural selection of homoiothermal animals in the process of evolution. Scand J Immunol 33:755–762. https://doi.org/10.1111/j.1365-3083.1991.tb02550.x
Tischenko VM, Abramov VM, Zav’yalov VP (1998) Investigation of the cooperative structure of Fc fragments from myeloma immunoglobulin G. Biochemistry 37:5576–5581. https://doi.org/10.1021/bi972647a
Ionescu RM, Vlasak J, Price C, Kirchmeier M (2008) Contribution of variable domains to the stability of humanized IgG1 monoclonal antibodies. J Pharm Sci 97:1414–1426. https://doi.org/10.1002/jps.21104
Nemergut M, Žoldák G, Schaefer JV et al (2017) Analysis of IgG kinetic stability by differential scanning calorimetry, probe fluorescence and light scattering. Protein Sci Publ Protein Soc 26:2229–2239. https://doi.org/10.1002/pro.3278
Klein C, Sustmann C, Thomas M et al (2012) Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs 4:653–663. https://doi.org/10.4161/mabs.21379
Fernández-Quintero ML, Quoika PK, Wedl FS et al (2022) Comparing antibody interfaces to inform rational design of new antibody formats. Front Mol Biosci 9:812750
Kahler U, Kamenik AS, Waibl F et al (2020) Protein–protein binding as a two-step mechanism: preselection of encounter poses during the binding of BPTI and trypsin. Biophys J 119:652–666. https://doi.org/10.1016/j.bpj.2020.06.032
Garber E, Demarest SJ (2007) A broad range of Fab stabilities within a host of therapeutic IgGs. Biochem Biophys Res Commun 355:751–757. https://doi.org/10.1016/j.bbrc.2007.02.042
Ewert S, Huber T, Honegger A, Plückthun A (2003) Biophysical properties of human antibody variable domains. J Mol Biol 325:531–553. https://doi.org/10.1016/S0022-2836(02)01237-8
Tsuchiya Y, Mizuguchi K (2016) The diversity of H3 loops determines the antigen-binding tendencies of antibody CDR loops. Protein Sci Publ Protein Soc 25:815–825. https://doi.org/10.1002/pro.2874
MacCallum RM, Martin AC, Thornton JM (1996) Antibody–antigen interactions: contact analysis and binding site topography. J Mol Biol 262:732–745. https://doi.org/10.1006/jmbi.1996.0548
Fernández-Quintero ML, Kraml J, Georges G, Liedl KR (2019) CDR-H3 loop ensemble in solution—conformational selection upon antibody binding. MAbs 11:1077–1088. https://doi.org/10.1080/19420862.2019.1618676
Fernández-Quintero ML, Loeffler JR, Kraml J et al (2018) Characterizing the diversity of the CDR-H3 loop conformational ensembles in relationship to antibody binding properties. Front Immunol 9:3065. https://doi.org/10.3389/fimmu.2018.03065
Fernández-Quintero ML, Math BA, Loeffler JR, Liedl KR (2019) Transitions of CDR-L3 loop canonical cluster conformations on the micro-to-millisecond timescale. Front Immunol. https://doi.org/10.3389/fimmu.2019.02652
Kuroda D, Shirai H, Kobori M, Nakamura H (2009) Systematic classification of CDR-L3 in antibodies: implications of the light chain subtypes and the VL–VH interface. Proteins 75:139–146. https://doi.org/10.1002/prot.22230
Giles I, Lambrianides N, Latchman D et al (2005) The critical role of arginine residues in the binding of human monoclonal antibodies to cardiolipin. Arthritis Res Ther 7:R47–R56. https://doi.org/10.1186/ar1449
Giles IP, Haley JD, Nagl S et al (2003) A systematic analysis of sequences of human antiphospholipid and anti-β2-glycoprotein I antibodies: the importance of somatic mutations and certain sequence motifs. Semin Arthritis Rheum 32:246–265. https://doi.org/10.1053/sarh.2003.49994
Birtalan S, Zhang Y, Fellouse FA et al (2008) The intrinsic contributions of tyrosine, serine, glycine and arginine to the affinity and specificity of antibodies. J Mol Biol 377:1518–1528. https://doi.org/10.1016/j.jmb.2008.01.093
Brockmann E-C, Pyykkö M, Hannula H et al (2021) Combinatorial mutagenesis with alternative CDR-L1 and -H2 loop lengths contributes to affinity maturation of antibodies. N Biotechnol 60:173–182. https://doi.org/10.1016/j.nbt.2020.09.002
Clark L, Boriack-Sjodin P, Day E et al (2009) An antibody loop replacement design feasibility study and a loop-swapped dimer structure. Protein Eng Des Sel 22:93–101. https://doi.org/10.1093/protein/gzn072
Geiger T, Clarke S (1987) Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem 262:785–794
Sydow JF, Lipsmeier F, Larraillet V et al (2014) structure-based prediction of asparagine and aspartate degradation sites in antibody variable regions. PLoS ONE. https://doi.org/10.1371/journal.pone.0100736
Fernández-Quintero ML, Heiss MC, Pomarici ND et al (2020) Antibody CDR loops as ensembles in solution vs. canonical clusters from X-ray structures. MAbs 12:1744328. https://doi.org/10.1080/19420862.2020.1744328
Zhou Z-H, Tzioufas AG, Notkins AL (2007) Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun 29:219–228. https://doi.org/10.1016/j.jaut.2007.07.015
Gunti S, Notkins AL (2015) Polyreactive antibodies: function and quantification. J Infect Dis 212:S42–S46. https://doi.org/10.1093/infdis/jiu512
Chiu ML, Goulet DR, Teplyakov A, Gilliland GL (2019) Antibody structure and function: the basis for engineering therapeutics. Antibodies 8:55. https://doi.org/10.3390/antib8040055
Horn JR, Sosnick TR, Kossiakoff AA (2009) Principal determinants leading to transition state formation of a protein–protein complex, orientation trumps side-chain interactions. Proc Natl Acad Sci USA 106:2559–2564. https://doi.org/10.1073/pnas.0809800106
Schaefer W, Regula JT, Bähner M et al (2011) Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci USA 108:11187–11192. https://doi.org/10.1073/pnas.1019002108
Regula JT, Imhof-Jung S, Mølhøj M et al (2018) Variable heavy-variable light domain and Fab-arm cross mAbs with charged residue exchanges to enforce correct light chain assembly. Protein Eng Des Sel 31:289–299. https://doi.org/10.1093/protein/gzy021
Codina N, Zhang C, Chakroun N, Dalby PA (2019) Insights into the stability of a therapeutic antibody Fab fragment by molecular dynamics and its stabilization by computational design. Biophysics. https://doi.org/10.1101/644369
Toughiri R, Wu X, Ruiz D et al (2016) Comparing domain interactions within antibody Fabs with kappa and lambda light chains. MAbs 8:1276–1285. https://doi.org/10.1080/19420862.2016.1214785
Acknowledgements
N.D.P. has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie Grant Agreement number 847476. The views and opinions expressed herein do not necessarily reflect those of the European Commission. Financial support by the European Union’s Horizon 2020 Research and Innovation Programme to P.K.Q. under Agreement Number 764958 is acknowledged. This work was supported by the Austrian Science Fund (FWF) via the Grants P34518 and P30737 and DOC30. The computational results have been partially achieved using the Vienna Scientific Cluster (VSC). We acknowledge PRACE for awarding us to access to Piz Daint at CSCS, Switzerland.
Funding
Open access funding provided by Austrian Science Fund (FWF). This study was supported by H2020 Marie Skodowska-Curie Actions; Austrian Science Fund (FWF); University of Innsbruck.
Author information
Authors and Affiliations
Contributions
NDP conducted research. FW and PKQ helped with data analysis and discussion of the results. GG, AB, MFQ and KRL contributed to the study conception and design, discussion of the results and revision of the manuscript. The first draft of the manuscript was written by NDP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
G.G. and A.B. are Roche employees and own Roche stocks; Roche has an interest in developing antibody-based therapeutics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pomarici, N.D., Waibl, F., Quoika, P.K. et al. Structural mechanism of Fab domain dissociation as a measure of interface stability. J Comput Aided Mol Des 37, 201–215 (2023). https://doi.org/10.1007/s10822-023-00501-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-023-00501-9