Abstract
Availability of COVID-19 mRNA vaccine for patients with chronic inflammatory demyelinating polyneuropathy (CIDP) treated with intravenous immunoglobulin (IVIg) raises the question of whether COVID-19 mRNA vaccine influences disease activity or IVIg-mediated immunomodulation in CIDP. In this exploratory study, blood samples of CIDP patients on IVIg treatment were longitudinally analyzed before and after vaccination with a COVID-19 mRNA vaccine. A total of 44 samples of eleven patients were characterized at four timepoints by ELISA and flow cytometry in terms of immunomarkers for disease activity and IVIg-immunomodulation. Apart from a significantly lower expression of CD32b on naïve B cells after vaccination, no significant alteration of immunomarkers for CIDP or IVIg-mediated immunomodulation was observed. Our exploratory study suggests that COVID-19 mRNA vaccine does not have a relevant impact on immune activity in CIDP. In addition, immunomodulatory effects of IVIg in CIDP are not altered by COVID-19 mRNA vaccine. This study was registered in the German clinical trial register (DRKS00025759).
Graphical Abstract
Overview over the study design. Blood samples of CIDP patients on recurrent IVIg treatment and vaccination with a COVID-19 mRNA vaccine were obtained at four timepoints for cytokine ELISA and flow cytometry, to assess key cytokines and cellular immunomarkers for disease activity and IVIg-immunomodulation in CIDP.

Similar content being viewed by others
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a prototypic autoimmune disease of the peripheral nervous system (Lehmann et al. 2019). Pathomechanisms of CIDP include autoreactive T cells (Chi et al. 2010), impaired B cell maturation and reduced expression of regulatory Fc gamma receptor IIb (FcγRIIb, CD32b) on B cells and monocytes, promoting autoantibody production against peripheral nerve myelin and phagocytosis (Press et al. 2003; Tackenberg et al. 2009). Over the last years, several studies tried to establish markers of peripheral blood mononuclear cells (PBMC) and serum cytokines that might reflect disease activity in CIDP. Of those, reduced numbers of CD32b expressing memory B cells and monocytes, as well as increased numbers of CD16+ myeloid dendritic cells, were associated with disease activity and/or poor response to immunoglobulin therapy (Tackenberg et al. 2009; Dyer et al. 2016). Other studies demonstrated that CIDP is associated with a reduction of regulatory T cells, as well as with an increase of monocytes (Matà et al. 2007; Sanvito et al. 2009).
Intravenous immunoglobulin (IVIg) is a first-line treatment for CIDP (Van den Bergh et al. 2021). IVIg attenuates CIDP-mediated autoimmunity via anti-idiotypic antibodies (Svačina et al. 2019), reduction of pro-inflammatory cytokines and chemokines (i.e., interleukin-6 [IL-6], or macrophage inflammatory protein-1α [MIP-1α], Hartung 2008; Ritter et al. 2014; Kajii et al. 2014), and by enhancing the expression of CD32b receptors on B cells and monocytes (Tackenberg et al. 2009). The effects of IVIg on CD32b expression are time-dependent. Whereas one week after IVIg infusion, CD32b expression decreases on B cells and monocytes due to IVIg-induced receptor internalization (Bouhlal et al. 2014; Dyer et al. 2016), Tackenberg and colleagues demonstrated that CD32b expression on these cells increases two to three weeks afterward (Tackenberg et al. 2009). Furthermore, IVIg suppresses pro-inflammatory myeloid dendritic cells and their pro-inflammatory CD32a (FcγRIIa) receptors (Boruchov et al. 2005; Dyer et al. 2016).
COVID-19 mRNA vaccine is known to provoke an immune response characterized by a transient increase of pro-inflammatory cytokines (e.g., IL-6), resulting in a SARS-CoV-2 specific proliferation of T cells, B cells, and anti-SARS-CoV-2 antibody-producing plasma cells (Goel et al. 2021; Jordan 2021). In contrast, whole cell counts of naïve, non-naïve, or memory B cells are considered to remain stable across the time course of vaccination (Goel et al. 2021).
Possible adverse effects of COVID-19 mRNA vaccine, like aseptic (peri-)myocarditis, might be caused by a misdirected, possibly stimulus-independent immune response after vaccination, but their exact pathomechanism is currently unknown (Vidula et al. 2021).
It is thus conceivable, yet unknown that COVID-19 mRNA vaccine alters the aberrant immune response in CIDP or impacts the immunomodulatory effects of IVIg. Therefore we here longitudinally examined for the first time the expression of selected cellular and humoral immunomarkers of CIDP and of IVIg-immunomodulation during the vaccination with a COVD-19 mRNA vaccine.
Methods
Patient Characteristics and Study Design
Eleven patients (9 male / 2 female, mean age 62 ± 17 years) with CIDP participated in this study. Inclusion criteria were confirmed or probable CIDP (according to the EFNS/PNS criteria (Van Den Bergh et al. 2010)) on recurrent IVIg treatment (every four weeks), the absence of autoantibodies against paranodal junction proteins, vaccination with a COVID-19 mRNA vaccine (Pfizer-BioNTech BNT162b2), and written informed consent for study participation. Exclusion criteria were acute infections or intake of immunosuppressants or systemic corticosteroids within the previous three months. Mean baseline INCAT disability score was 2 ± 2, mean baseline Rasch-built overall disability scale (R-ODS) score was 73 ± 19.
Blood serum samples and peripheral blood mononuclear cell (PBMC) samples were collected at four timepoints: Before vaccination (mean 11 ± 4 weeks before the first vaccine dose), samples were collected before (1st, on the same day) and one week after IVIg infusion (2nd). The mean interval between the first and the second dose of COVID-19 vaccine was 4 ± 1 weeks. Sample collection was repeated two weeks after the second COVID-19 vaccine dose immediately before (3rd) and one week after IVIg infusion (4th).
Sample Preparation
Blood samples were collected by venipuncture using Sarstedt S-monovettes® (Sarstedt, Nümbrecht, Germany) for serum collection and the BD Vacutainer CPT™ system (BD Biosciences, San Diego, CA, USA) for PBMC isolation.
Serum samples were obtained by centrifugation of whole blood monovettes at 3000 rotations per minute (rpm) for 10 min and then stored at -20 °C for further use.
PBMCs were isolated from the BD Vacutainer CPT™ system according to the manufacturers ‘ instructions before being stored at -80 °C (15 million PBMCs in 100 µl dimethylsulfoxide [DMSO] and 900 µl fetal bovine serum [FBS]).
Immunophenotyping by Flow Cytometry
The following antibodies were used for the analysis of peripheral B and T lymphocytes, monocytes, and myeloid dendritic cells by flow cytometry: CD3 (OKT3)-BV650, CD11c (3.9)-BV510, CD14 (63D3)-PE, CD16 (3G8)-APC/Cy7, CD19 (HIB19)-PE/Cy7, CD27 (O323)-BV785, CD32b/c (S18005H)-APC, CD56 (5.1H11)-PE Dazzle 594, all from Biolegend, San Diego, CA, USA, and CD32a (#2)-FITC (SinoBiological, Beijing, China).
Raw flow cytometry data were acquired on a BD LSR Fortessa™ cytometer (BD Biosciences) and data were analyzed using FlowJo v.10.8.0 software (BD Biosciences).
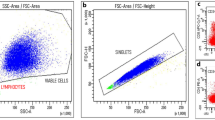
All flow cytometry experiments included isotype controls and fluorescence-minus-one controls. Doublet cells were excluded from analyses based on forward scatter-A and forward scatter-H. For each experiment, the lymphocyte and the monocyte population were gated using forward and side scatter.
Monocytes were characterized by excluding CD3 and CD19 positive cells and the differential expression of CD14 and CD16, and defined as classical (CD14+CD16−) and non-classical (CD14dimCD16+) monocytes. Thereafter, the expression of pro-inflammatory CD32a or anti-inflammatory CD32b on monocytes was assessed. The whole lymphocyte population was differentiated into T cells (CD3+CD32a+ and CD3+CD32b+), naïve B cells (CD19+CD27−), either expressing CD32a (CD19+CD27−CD32a+) or CD32b (CD19+CD27−CD32b+), or memory B cells expressing the same antigens (CD19+CD27+CD32a+, CD19+CD27+CD32b+). As CD32c is uniquely expressed on NK cells (Veri et al. 2007), only CD32b expression was considered on the cell populations analyzed in this study.
Myeloid dendritic cells (mDCs) were defined as CD11c+ live cells that were negative for CD3, CD14, CD19, and CD56 (Suppl. Figure 1).
Serum Cytokine ELISA
Serum samples were analyzed for IL-6, IL-10, IL-33, MIP-1α, and TGF-β concentrations using sandwich enzyme-linked immunosorbent assay (ELISA) kits (ImmunoTools, Friesoythe, Germany, for IL-6 and IL-10) and Invitrogen human IL-33, MIP-1α, and TGF-β sandwich ELISA kits (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturers ‘ instructions. Plates were read at 450 nm and optical densities were analyzed using the BMG Labtech MARS® software (BMG Labtech, Ortenberg, Germany). Serum cytokine concentrations were calculated by interpolating the individual sample’s optical density with a standard concentration curve.
Statistical Analysis
Statistical analysis was performed using GraphPad PRISM 9.0 software (Graphpad, San Diego, CA, USA). Normality was tested using D’Agostino and Pearson omnibus normality test, before either multiple paired t-tests or Wilcoxon tests were performed. A p-value < 0.05 was considered statistically significant.
Results
All CIDP patients showed relevant IgG reactivity against SARS-CoV-2 two weeks after the second dose of COVID-19 mRNA vaccine, which was measured by chemiluminescent microparticle immunoassay (CMIA, mean anti-SARS-CoV-2 IgG serum titer: 1670 ± 914 BAU/ml).
-
1.
Immunomarkers of CIDP disease activity before and after COVID-19 vaccination
CD32b expression on naïve B cells was lower in post vaccination samples (11.2 ± 4.9% vs. 43.8 ± 17.8%, p = 0.001; Fig. 1A), whereas no significant difference was seen on memory B cells (28.2 ± 17.8% vs. 10.6 ± 6.9%, p = 0.06). Contrariwise, in post vaccination samples, CD32a expression on naïve (18.9 ± 10.3% vs. 5.8 ± 4.2%, p = 0.008) and memory (14.7 ± 12.4% vs. 1.3 ± 1.1%, p = 0.03) B cells was higher compared to samples before vaccination (Fig. 1B-C).
The whole memory B cell fraction (CD19+CD27+) was not different in samples before and after vaccination (28.0 ± 15.4% vs. 28.4 ± 17.4%, p = 0.81; Fig. 1D). Furthermore, COVID-19 vaccination did not alter CD32b expression on classical monocytes (10.2 ± 6.8% vs. 19.6 ± 11.7%, p = 0.25; Fig. 1E).
-
2.
Immunomarkers of IVIg-immunomodulation before and after COVID-19 vaccination
IVIg administration significantly reduced CD32b expression on naïve B cells before (19.4 ± 11.5% vs. 43.8 ± 17.8%, p= 0.02; Fig. 1A) and after vaccination (9.2 ± 2.3% vs. 11.2 ± 4.9%, p = 0.03; Fig. 1A), whereas CD32b expression on memory B cells was not significantly changed by IVIg treatment (28.2 ± 17.8% vs. 11.3 ± 23%, p= 0.30). IVIg administration did not alter CD32a expression on naïve and memory B cells before (p = 0.16 [naïve B cells], p = 0.66 [memory B cells]) and after (p = 0.25 [naïve B cells], p = 0.06 [memory B cells]) COVID-19 vaccination (Fig. 1B-C).
IVIg administration resulted in a lower memory B cell amount in vaccine-naïve samples (8.5 ± 8.1% vs. 28.4 ± 17.4%, p = 0.03), but not after COVID-19 vaccination (Fig. 1D). Furthermore, IVIg administration led to a significant increase of CD32b expression on classical monocytes after COVID-19 vaccination (12.6 ± 9.6% vs. 10.2 ± 6.8%, p = 0.02; Fig. 1E).
The amount of myeloid dendritic cells (mDC) was significantly lower after COVID-19 vaccination and IVIg administration compared to samples prior to vaccination (1.8 ± 1.6% vs. 17.5 ± 24.4%, p = 0.02; Fig. 1F). Finally, COVID-19 vaccination and IVIg administration similarly led to a sustained decrease of CD32b expressing T cells (0.1 ± 0.1% vs. 16.4 ± 19.4%, p = 0.008) and non-classical monocytes (0% vs. 2.3 ± 3.1%, p = 0.03).
-
3.
Serum cytokine levels before and after COVID-19 vaccination
IL-6 was increased in serum samples after IVIg administration before and after COVID-19 vaccination (Δ IL-6 [post IVIg – pre IVIg] after vs. prior to COVID-19 vaccination [in pg/ml]: 11.0 ± 7.4 vs. 2.3 ± 1.9, p = 0.01), whereas the absolute serum concentrations were comparable (17.6 ± 23.6 pg/ml vs. 17.6 ± 24.2 pg/ml; Fig. 2A).
Serum concentration differences related to IVIg administration (Δ [post IVIg – pre IVIg]) of IL-10 (381.2 ± 295.5 pg/ml vs. 240.5 ± 173.3 pg/ml, p = 0.35; Fig. 2B), of IL-33 (0.07 ± 0.2 pg/ml vs. 0.52 ± 1.1 pg/ml, p = 0.21; Fig. 2C), of MIP-1α (8.2 ± 8.6 pg/ml vs. 5.9 ± 3.6 pg/ml, p = 0.82; Fig. 2D), and of TGF-β (4147 ± 2567 pg/ml vs. 2826 ± 2217 pg/ml, p = 0.39; Fig. 2E) were not different before and after COVID-19 vaccination. Likewise, absolute serum concentrations of these cytokines were not relevantly altered by COVID-19 vaccination (Fig. 2A-E).
-
4.
Disability before and after COVID-19 vaccination
No changes in disability, measured by the INCAT disability scale (mean INCAT score before and after vaccination: 2 ± 2) and the R-ODS score (mean R-ODS score before and after vaccination: 73 ± 19), occurred after COVID-19 vaccination. Also, no subjective clinical deterioration was reported from CIDP patients after COVID-19 vaccination.
Alterations of immune cell fractions after IVIg administration before and after COVID-19 vaccination. CD32b expression on naïve B cells is significantly reduced after IVIg administration and after COVID-19 vaccination (A). Contrariwise, CD32a expression on naïve and memory B cells increases after COVID-19 vaccination (B,C). IVIg administration significantly decreases CD19+CD27+ memory B cells in an unvaccinated state, but not after COVID-19 vaccination (D). CD32b expression on classical monocytes is increased after IVIg administration and vaccination (E). IVIg administration tends to reduce CD11c+ myeloid dendritic cells, with a significantly reduced cell amount after COVID-19 vaccination (F). Blue arrow = Second dose of COVID-19 mRNA vaccine. * p < 0.05, **p < 0.01
Alterations of serum cytokine concentrations after IVIg administration and COVID-19 vaccination. The relative increase of IL-6 concentrations after IVIg administration is significantly higher after COVID-19 vaccination than in an unvaccinated state, whereas the absolute concentrations remain stable (A). IL-10 concentrations significantly increase after IVIg administration and COVID-19 vaccination, but no significant difference in relative increase is seen after vaccination compared to an unvaccinated state (B) IL-33 and MIP-1α concentrations are not significantly altered by IVIg administration or COVID-19 vaccination (C,D). TGF-β concentrations decrease after IVIg administration, without a relevant influence of COVID-19 vaccination on the extent of the relative decrease (E). Blue arrow = Second dose of COVID-19 mRNA vaccine. *p < 0.05
Discussion
To our knowledge, this is the first study that explored a set of presumed markers of disease activity and IVIg- immunomodulation in CIDP patients who underwent vaccination with the novel mRNA vaccine BNT162b2. As such, our study provides important insights into the vaccine response in a chronic autoimmune condition. Our data indicate that in CIDP, immunomarkers for disease activity and IVIg- immunomodulation were not relevantly altered by COVID-19 mRNA vaccine.
The most striking difference in the expression of disease activity markers before and after vaccination was the lower expression of anti-inflammatory CD32b on naïve B cells and an increased expression of pro-inflammatory CD32a. CD32b (FcγRIIb) is known to control antibody generation upon immunization (Takai et al. 1996). As such, it appears attractive to speculate that this decline could be a systemic vaccine effect. However, this hypothesis would need confirmation by similar observations in other IVIg-dependent and -independent conditions. The reduction of CD32b on naïve B cells could be related to CD32a upregulation, as these two immunoglobulin receptors mediate opposing functions on B cells, probably leading to a relative CD32b downregulation when CD32a is upregulated (Nimmerjahn and Ravetch 2007). Regarding its function for the pathogenesis of CIDP, we assume that the decrease of CD32b on naïve B cells is not clinically relevant for the following reasons: 1) CD32b is known to decline subsequent to IVIg treatment on naïve B cells in CIDP within the first week (Dyer et al. 2016). 2) CD32b expression on memory B cells—which are also considered to be critical for CIDP and its treatment with IVIg (Tackenberg et al. 2009)—was not altered. 3) None of the patients experienced any clinical deterioration upon the vaccination.
Also, the activation of pro-inflammatory CD32a receptors on B cells may reflect a physiological activation of the humoral adaptive immune system for anti-SARS-CoV-2 antibody production, as studies on pneumococcal vaccine showed that CD32a activation plays a crucial role for vaccine response, promoting vaccine-induced antibody generation (Wiertsema et al. 2006). Regarding its role in terms of mediating IVIg efficacy, it has to be noted that although CD32a is known to be suppressed by IVIg, our data and a previous study indicate that this mechanism of action does not seem to play a major role in the pathogenesis in CIDP (Quast et al. 2015). Our observation that none of our patients progressed or relapsed upon the vaccination further supports this conclusion.
Furthermore, other markers for IVIg-immunomodulation in CIDP, like increasing CD32b expression on classical monocytes (Tackenberg et al. 2009) and a decrease of myeloid dendritic cells (Dyer et al. 2016), were not altered after COVID-19 vaccination, further supporting the assumption that COVID-19 mRNA vaccine does not have a relevant impact on immunomarkers for CIDP.
To our knowledge, this study is also the first that explored potential changes in serum cytokine levels subsequent to the vaccination with a COVID-19 mRNA vaccine. Our findings that most of the assessed cytokines showed comparable expression profiles in paired serum samples before and after vaccination further support the notion that the vaccine overall does not impact markers of systemic inflammation. The slightly stronger increase of IL-6 serum levels post IVIg after COVID-19 vaccination could reflect a vaccine-specific immune response (Jordan 2021). This hypothesis is indirectly supported by a recent study that demonstrated that patients under anti-IL6 treatment showed lower antibody responses to COVID-19 mRNA vaccine (Picchianti-Diamanti et al. 2021). Whether our exploratory findings are also transferable to other autoimmune diseases has to be explored by future studies, and recent reviews focusing on COVID-19 vaccination and its potential to influence the course of Guillain-Barré syndrome or systemic lupus erythematosus demonstrate that such studies would raise potential interest (Mason et al. 2021; Lahoz Fernandez et al. 2022). Furthermore, vaccine selection decision-making models might be useful to minimize adverse vaccine effects in patients with autoimmune diseases, as they present with different host factors and a possibly different susceptibility to adverse immunological side effects than the average population (Abdelwahab et al. 2021; Mason et al. 2021).
A recent study demonstrated that anti-SARS-CoV-2 antibody generation is not directly altered by IVIg in CIDP, and that a sufficient vaccine efficacy is assumable in CIDP patients, although CIDP-specific immunological host factors have to be considered (Svačina et al. 2022).
Our study has several limitations, as the small cohort size of eleven patients only provides first exploratory data about the interaction of COVID-19 mRNA vaccine with immunomarkers of CIDP and IVIg-immunomodulation, that will require confirmation by larger follow-up studies also including untreated CIDP patients or unvaccinated IVIg recipients as control cohorts, as well as longitudinal data about the effects of booster vaccinations, that were lacking in our study. Secondly, as COVID-19 mRNA vaccine prevents SARS-CoV-2 from entering human cells via the angiotensin-converting enzyme 2 receptor (ACE2R; Gattinger et al. 2022), and as data about a differential expression of ACE2R in CIDP and healthy subjects, that could generally influence vaccine efficacy in CIDP, are currently lacking, future studies also will have to elucidate ACE2R expression in CIDP. Thirdly, our study did not evaluate the response of CD8-positive cyctotoxic T cells or plasma cells to COVID-19 mRNA vaccine due to its focus on distinct immunomarkers of CIDP. As cyctotoxic T cells and plasma cells play an important role by executing vaccine-induced SARS-CoV-2 immunity (Ssemaganda et al. 2022), future studies should also focus on vaccine-induced alterations of these cell populations in CIDP. On the other hand, our study evaluated CD32a/b expression on B cells, that either enhance or reduce plasma cell-mediated antibody generation (Karnell et al. 2014), therefore a concordance of post-vaccine plasma cell alterations to the CD32 receptor expression patterns observed in our study appears assumable.
In summary, considering the exploratory approach of our study and all its limitations, our study suggests that neither the examined immune mechanisms in CIDP nor key immunomodulatory pathways of IVIg are significantly altered two weeks after full COVID-19 immunization, which is a timepoint assumed to be safe for post-vaccine IVIg administration by expert opinions, but was so far lacking confirmative data (Doneddu et al. 2021).
Data Availability
The data of this study are not publicly available due to ethical restrictions. They may be made available on reasonable request (e.g., for replicating procedures and results) by the corresponding author after consultation with the co-authors.
Abbreviations
- ACE2R :
-
angiotensin-converting enzyme receptor 2
- CD :
-
cluster of differentiation
- CIDP :
-
chronic inflammatory demyelinating polyneuropathy
- COVID-19 :
-
coronavirus disease 19
- DMSO :
-
dimethylsulfoxide
- ELISA :
-
enzyme-linked immunosorbent assay
- FACS :
-
fluorescence activated cell sorting
- FBS :
-
fetal bovine serum
- FcγR :
-
Fc gamma receptor
- IgG :
-
immunoglobulin G
- IVIg :
-
intravenous immunoglobulin
- mDC :
-
myeloid dendritic cell
- ml :
-
milliliter
- PBMC :
-
peripheral blood mononuclear cells
- PBS :
-
phosphate buffered saline
- rpm :
-
rotations per minute
- SARS-CoV-2 :
-
severe acute respiratory syndrome coronavirus 2
- μl :
-
microliter
References
Abdelwahab SF, Issa UH, Ashour HM (2021) A novel vaccine selection decision-making model (Vsdmm) for covid-19. Vaccines 9
Boruchov AM, Heller G, Veri MC et al (2005) Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest 115:2914–2923
Bouhlal H, Martinvalet D, Teillaud JL et al (2014) Natural autoantibodies to Fcγ receptors in intravenous immunoglobulins. J Clin Immunol 34
Chi LJ, Xu WH, Zhang ZW et al (2010) Distribution of Th17 cells and Th1 cells in peripheral blood and cerebrospinal fluid in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst 15:345–356
Doneddu PE, Spina E, Briani C et al (2021) Acute and chronic inflammatory neuropathies and COVID-19 vaccines: Practical recommendations from the task force of the Italian Peripheral Nervous System Association (ASNP). J Peripher Nerv Syst 26:148–154
Dyer WB, Tan JCG, Day T et al (2016) Immunomodulation of inflammatory leukocyte markers during intravenous immunoglobulin treatment associated with clinical efficacy in chronic inflammatory demyelinating polyradiculoneuropathy. Brain Behav 6
Gattinger P, Niespodziana K, Stiasny K et al (2022) Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy Eur J Allergy Clin Immunol 77:230–242
Goel RR, Apostolidis SA, Painter MM et al (2021) Distinct antibody and memory B cell responses in SARSCoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol 6:1–19
Hartung HP (2008) Advances in the understanding of the mechanism of action of IVIg. J Neurol 255:3–6
Jordan SC (2021) Innate and adaptive immune responses to SARS-CoV-2 in humans: relevance to acquired immunity and vaccine responses. Clin Exp Immunol 204:310–320
Kajii M, Kobayashi F, Kashihara J et al (2014) Intravenous immunoglobulin preparation attenuates neurological signs in rat experimental autoimmune neuritis with the suppression of macrophage inflammatory protein -1α expression. J Neuroimmunol 266:43–48
Karnell JL, Dimasi N, Karnell FG et al (2014) CD19 and CD32b Differentially Regulate Human B Cell Responsiveness. J Immunol 192:1480–1490
Lahoz Fernandez PE, Miranda Pereira J, Fonseca Risso I et al (2022) Guillain-Barre syndrome following COVID-19 vaccines: A scoping review. Acta Neurol Scand 145:393–398
Lehmann HC, Burke D, Kuwabara S (2019) Chronic inflammatory demyelinating polyneuropathy: Update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry 90:981–987
Mason A, Anver H, Lwin M et al (2021) Lupus, vaccinations and COVID-19: What we know now. Lupus 30:1541–1552
Matà S, Corzani S, Biagiotti R et al (2007) Influence of impaired T- and B-cell compartments on efficacy of IVIg in dysimmune neuropathies. Eur J Neurol 14:1147–1153
Nimmerjahn F, Ravetch JV (2007) Fc-Receptors as Regulators of Immunity. Adv Immunol 96:179–204
Picchianti-Diamanti A, Aiello A, Laganà B et al (2021) Immunosuppressive Therapies Differently Modulate Humoral- and T-Cell-Specific Responses to COVID-19 mRNA Vaccine in Rheumatoid Arthritis Patients. Front Immunol 12:1
Press R, Pashenkov M, Jin JP, Link H (2003) Aberrated levels of cerebrospinal fluid chemokines in Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Clin Immunol 23:259–267
Quast I, Cueni F, Nimmerjahn F et al (2015) Deregulated Fcy receptor expression in patients with CIDP. Neurol Neuroimmunol NeuroInflammation 2:148
Ritter C, Förster D, Albrecht P et al (2014) IVIG regulates BAFF expression in patients with chronic inflammatory demyelinating polyneuropathy (CIDP). J Neuroimmunol 274:225–229
Sanvito L, Makowska A, Gregson N et al (2009) Circulating subsets and CD4CD25 regulatory T cell function in chronic inflammatory demyelinating polyradiculoneuropathy. Autoimmunity 42:667–677
Ssemaganda A, Nguyen HM, Nuhu F et al (2022) Expansion of cytotoxic tissue-resident CD8+ T cells and CCR6+CD161+ CD4+ T cells in the nasal mucosa following mRNA COVID-19 vaccination. Nat Commun 13
Svačina MKR, Meißner A, Schweitzer F et al (2022) Antibody response after COVID-19 vaccination in intravenous immunoglobulin-treated immune neuropathies. Eur J Neurol 29:3380–3388
Svačina MKR, Röth P, Bobylev I et al (2019) Changes of Serum IgG Dimer Levels after Treatment with IVIg in Guillain-Barré Syndrome. J Neuroimmune Pharmacol 14:642–648
Tackenberg B, Jelčić I, Baerenwaldt A et al (2009) Impaired inhibitory Fcγ receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci USA 106:4788–4792
Takai T, Ono M, Hikida M et al (1996) Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature 379:346–349
Van Den Bergh PYK, Hadden RDM, Bouche P et al (2010) European federation of neurological societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripher. Eur J Neurol 17:356–363
Van den Bergh PYK, van Doorn PA, Hadden RDM et al (2021) European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force—Second revision. J Peripher Nerv Syst 26:242–268
Veri MC, Gorlatov S, Li H et al (2007) Monoclonal antibodies capable of discriminating the human inhibitory Fcγ-receptor IIB (CD32B) from the activating Fcγ-receptor IIA (CD32A): Biochemical, biological and functional characterization. Immunology 121:392–404
Vidula MK, Ambrose M, Glassberg H et al (2021) Myocarditis and Other Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines. Cureus 13. https://doi.org/10.7759/cureus.15576
Wiertsema SP, Veenhoven RH, Walraven V et al (2006) Pneumococcal vaccine efficacy for mucosal pneumococcal infections depends on Fcγ receptor IIa polymorphism. Vaccine 24:792–797
Acknowledgements
The authors thank all patients for their study participation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MKRS designed and conceptualized the study, collected blood samples, designed the manuscript and its graphic material and revised the manuscript for intellectual content. AM helped conceptualizing the study, collected blood samples, and revised the manuscript for intellectual content. FS helped conceptualizing the study and revised the manuscript for intellectual content. ASS, IK, HW, FK, HLW and MS revised the manuscript for intellectual content. HCL designed and conceptualized the study, supervised the study conduction and revised the manuscript for intellectual content.
Corresponding author
Ethics declarations
Ethical Approval
The University of Cologne Ethics Committee approved the study (approval reference number: 19-1662_1). Furthermore, this study was registered in the German clinical trial register (DRKS00025759). It was carried out in line with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
All CIDP patients gave written informed consent for study participation.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Svačina, M.K.R., Meißner, A., Schweitzer, F. et al. CIDP: Analysis of Immunomarkers During COVID-19 mRNA-Vaccination and IVIg-Immunomodulation: An Exploratory Study. J Neuroimmune Pharmacol 18, 208–214 (2023). https://doi.org/10.1007/s11481-023-10058-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-023-10058-x