Abstract
Pain has a multifaceted impact on individuals worldwide, affecting their physical functioning, emotional well-being, and quality of life. Children (age < 18 years) have a high prevalence of conditions associated with pain, such as toothache, headache, earache, sore throat, and respiratory tract infections, many of which may be accompanied by fever. Globally, the pharmacologic treatment of pain in pediatric patients is limited largely to nonopioid analgesics, and dosing must account for differences in age, weight, metabolism, and risk of adverse effects. This narrative review summarizes the findings of a literature search on the pediatric indications, dosing approaches, dosing guidelines, and pharmacokinetics of paracetamol and ibuprofen, which are common pain medications available globally for self-care use in children. The review also discusses the risks and benefits associated with these agents. The current roles of paracetamol and ibuprofen in the symptomatic management of coronavirus disease 2019 (COVID-19) infection and in the management of post–COVID-19 immunization symptoms in children are also discussed. Therefore, while a very large amount of data over several decades is available for paracetamol and ibuprofen, an urgent need exists for well-designed studies of these medications for the management of pain and fever in pediatric patients with COVID-19 to ensure optimal relief with minimal toxicity.
Similar content being viewed by others
Common childhood conditions such as teething, headache, ear infections, sore throat, and respiratory infections are often associated with pain and fever. |
The pharmacologic treatment of mild pain and fever in pediatric patients in self-care is limited to analgesics like paracetamol and ibuprofen in children > 3 months of age who can take oral medication or only paracetamol for children between 1 and 3 months of age. |
Age, weight, and drug distribution should be considered when determining the correct dose required to achieve a target concentration associated with analgesia or antipyresis. |
1 Introduction
Over 90% of individuals worldwide suffer from pain each year. The global burden of pain was demonstrated in a combined analysis of three international online surveys (editions 1, 2, and 3 of the Global Pain Index [GPI] study) carried out to quantify the perceived immediate impact of pain on individuals’ lives [1]. Responses of ~ 29,000 individuals from 14 countries who were ≥ 18 years of age and had ever experienced musculoskeletal pain were analyzed [1]. Results indicated that for roughly half of the survey population, the burden of pain involves pain frequency of at least once weekly, pain duration of at least several hours, decreased ability to be happy, reduced quality of life, and impaired ability to enjoy life.
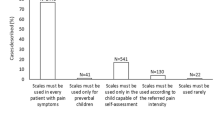
Pain is common among infants, children, and adolescents. Types of pain frequently experienced in pediatric populations are listed in Table 1 [2]. The fourth edition of the GPI study (GPI-4), which was carried out in 19 countries in 2020 to explore self-care of pain (includes parent-to-child treatment), included information on self-care of pain indications in children up to 18 years of age based on responses given by their parents. Results demonstrated that pain conditions with a prevalence of > 50% in children ages ≤ 18 years (as reported by 7917 parents) are toothache (53%), earache (54%), headache (54%), fever and pain associated with vaccination/immunization (59%), stomachache (64%), respiratory tract infections (71%), and sore throat (72%) (GPI-4 unpublished data). Findings also demonstrated the burden of managing a child’s pain on parents, with 73% reporting that their children are miserable or not their usual selves when they are in pain and prompting 40% to report feeling panicked and helpless [3].
Common pain medications available globally for use in children are paracetamol (acetaminophen) and nonsteroidal anti-inflammatory drugs (NSAIDs); in many low- and middle-income countries, limited access to medications leaves these as the only medications available. Because of safety, addiction, and diversion concerns, opioids are not typically used to treat mild to moderate pain in the pediatric population. Metamizole (dipyrone), a cyclooxygenase-1 and cyclooxygenase-2 (COX-1 and COX-2) inhibitor, is used in some countries for the treatment of pediatric pain conditions, despite the high risk for serious adverse effects, particularly agranulocytosis [4, 5]. Because of this risk, use of metamizole is banned in the United States and most Western European countries. A review of the literature concluded that no evidence existed to support the superiority of metamizole over paracetamol or ibuprofen for pediatric analgesia and that such use should not be recommended or encouraged [4].
Paracetamol acts to relieve pain and fever. Its analgesic effect is not completely understood but is believed to be a direct action of the metabolite N-acylphenolamine on the brain and dorsal horn of the spinal cord [6]. Its antipyretic effect is thought to involve central nervous system inhibition of a COX-1 protein variant and potential inhibition of COX-2, as well as peripheral nervous system inhibition of transient receptor potential subfamily ankyrin 1 channels on sensory neurons by the liver metabolites N-acetyl-p-benzoquinoneimine and p-benzoquinone [7]. The central and peripheral nervous systems are also targets for the analgesic effects of paracetamol [7].
NSAIDs (e.g., ibuprofen) act to relieve pain, fever, and inflammation in children over 3 months of age [8]. Their effects result from inhibition of prostaglandin production from arachidonic acid via inhibition of COX-1 and COX-2 [9]. COX-1 and COX-2 are also called prostaglandin synthase and they have binding sites for COX and peroxidase. COX-1 is responsible for regulating normal cell processes and is expressed in most tissues. In contrast, COX-2 is constitutively active in the brain, kidney, and bone but is normally not measurable in most other tissues [9]. Although both COX-1 and COX-2 lead to the production of prostaglandins, it is induction of COX-2 that leads to production of prostaglandins associated with inflammation. Nonselective NSAIDs inhibit both COX-1 and COX-2, while selective NSAIDs, also called COX-2 inhibitors, preferentially inhibit COX-2. However, data on the effects of COX-1/2 inhibition on inflammation are inconclusive [9].
The objectives of this narrative review are to summarize the findings of a literature search on common pediatric indications, dosing approaches, dosing guidelines, and pharmacokinetics of paracetamol and ibuprofen, common pain medications available for self-care use in children, and to discuss the associated risks and benefits. Their current roles in the symptomatic management of coronavirus disease 2019 (COVID-19) infection and in the management of post–COVID-19 immunization symptoms in children are also discussed.
2 Literature Analysis
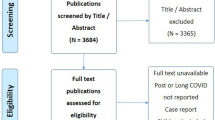
The search identified 72 relevant studies and reviews and 20 sets of guidelines. Additional articles (ten studies, three reviews) and guidelines (13 articles) were included at the discretion of the authors. Among the studies and reviews, 31 included common indications of paracetamol and 25 included common indications of ibuprofen. A summary of the key clinical studies and meta-analyses of paracetamol and ibuprofen use in pediatric patients is presented in Table 2.
A literature search was conducted to retrieve published articles and guidelines on the common indications and dosing approaches of paracetamol and ibuprofen in pediatric patients. Additional articles and guidelines were included at the discretion of the authors.
The ProQuest (Derwent Drug File, Embase, MEDLINE, ToxFile) database was searched for English-language articles published through September 2022 (strategy outlined in Supplementary Table 1, see the electronic supplementary material). Inclusion criteria were the following: clinical studies, systematic reviews, and review articles (meta-analyses) on the effectiveness and safety of paracetamol, ibuprofen, and NSAIDs in children; studies in common indications (e.g., fever, pain, migraine/headache, muscle ache, sore throat, musculoskeletal pain, fever and pain associated with vaccination/immunization, pain after dental procedures/tooth extraction, toothache, earache/otalgia, respiratory tract infections including cold and flu, COVID-19); and studies including medications with oral routes of administration (tablets/capsules/suspensions). Articles were excluded if they described studies in prescription indications, such as febrile seizures, lower abdominal surgeries, perineal surgeries, spinal surgeries, concussion (mild traumatic brain injury), and patent ductus arteriosus, if the studies did not use standard doses of paracetamol (10–15 mg/kg; 60 mg/kg/day) or ibuprofen (5–10 mg/kg; 30 mg/kg/day), if they were in a language other than English, or if they were conducted in adolescents and adults. Nonclinical studies, case reports, letters, comments, and editorials were also excluded.
To fully capture relevant safety-related pharmacokinetic data on paracetamol use in the pediatric population, we conducted additional searches on the clinical pharmacology of paracetamol pediatric formulations from the last 3 years (30 June 2019–30 September 2022; Supplemental Table 2).
Country-specific guidelines on the use of paracetamol and ibuprofen in the management of pediatric indications were reviewed to identify areas of consensus. Health authority guidelines (e.g., those from the Centers for Disease Control and Prevention [CDC], National Institutes of Health [NIH], and World Health Organization [WHO]) on the symptomatic management of COVID-19 infection in children and on the management of post-vaccine symptoms in children eligible to receive COVID-19 vaccines, as well as current prescribing information for the available COVID-19 vaccines (Pfizer-BioNTech [COMIRNATY], Moderna, Johnson & Johnson/Janssen, and Sinovac [CoronaVac] vaccines) were reviewed to provide information on the roles of paracetamol and ibuprofen in these applications.
3 Pharmacokinetics
3.1 Paracetamol
Paracetamol is a highly lipid-soluble compound with a maximum observed concentration (Cmax) of 12.6 µg/mL, a time to reach Cmax (tmax) of ~ 2 h, and an elimination half-life (t½) of ~ 2.3 h in children for the 15 mg/kg dose of its oral formulation [10]. Variations have been noted across formulations; for example, a t½ of 3.8 h has been recorded using paracetamol suppositories in neonates [11]. Oral paracetamol is rapidly absorbed with a mean systemic availability of ~ 75% [12]. Paracetamol is primarily eliminated by glucuronidation and sulfation pathways, which have been reported to be 55% and 30% of the total urinary excretion, respectively [12, 13]. However, some aspects of the pharmacokinetics of paracetamol vary by age owing to maturation processes and developmental growth [14,15,16,17]. For example, absorption is slower (longer time to Cmax) and clearance increases exponentially over the first 12 months of life (4.9 L/h/70 kg at birth to 12.4 L/h/70 kg by 12 months) [15]. The clearance in infants is reported to be 80% that of a 2-year-old child by 6 months of age [15]. In a population pharmacokinetic analysis of paracetamol in premature neonates and infants, the volume of distribution decreased exponentially from 109.7 L/70 kg at 28 weeks to 72.9 L/70 kg by 60 weeks after conception, with a maturation half-life of 11.5 weeks [16]. Clearance increased from 0.74 L/h/70 kg at 28 weeks to 10.8 L/h/70 kg at 60 weeks, with a maturation half-life of 11.3 weeks [16]. In addition, t½ is longer for neonates than for older children. The t½ of paracetamol is 2.5–4 h in neonates, 11 h for 28- to 32-week-old neonates (for rectally administered paracetamol), and 4–5 h in 32- to 36-week-old neonates [14]. A relationship between glucuronide clearance and age has been suggested in neonates and children, wherein glucuronide clearance has been shown to increase with age [14]. This is expected, as the sulfation pathways mature at birth, while the glucuronidation pathways mature around 2 years of age [14]. In addition to the two phase II metabolic pathways, a small proportion (~ 2%) of unchanged paracetamol is eliminated in the urine [18]. The remainder (~ 10%) undergoes phase I oxidation by hepatic cytochrome P450 2E1 (CYP2E1) (and to a lesser extent with CYP1A2 and CYP3A4), which leads to the formation of a highly reactive toxic metabolite, N-acetyl-para-benzo-quinone imine (NAPQI) [18]. Evidence suggests that the hepatotoxicity associated with paracetamol overdose may occur through the formation of the toxic NAPQI metabolite, which has been detected in excessive levels in those exposed to very high doses of paracetamol [18]. The excessive formation of NAPQI leads to glutathione depletion, oxidative stress, and mitochondrial dysfunction, ultimately resulting in the cessation of adenosine triphosphate (ATP) synthesis [18]. However, large-scale studies are required to correlate these findings and to understand the link between the toxic and analgesic effects of paracetamol with specific genotypes in order to improve the benefit–risk ratio of paracetamol with adapted pharmacogenetic screening [19].
In addition to age, body weight is another factor accounting for variation in the pharmacokinetics of drugs that undergo hepatic metabolism, such as paracetamol [20, 21]. When comparing standard weight-based dosing regimens of paracetamol, clearance increases from birth through childhood. Two studies showed that the dosing for obese or overweight children required significantly higher paracetamol dosages; however, the dosages received were either lower or even higher than the recommended dosage (10–15 mg/day), without necessary dose alteration, compared with children of healthy weight [22, 23]. A multicenter prospective observational cohort study in the United Kingdom found that the median (interquartile range) dose of oral paracetamol (mg/kg) was significantly lower (P < 0.001) for obese versus non-obese children 2–16 years of age, despite obese children requiring significantly greater analgesia more frequently in the post-anesthetic care unit than children of healthy weight (P = 0.04) [22]. In contrast, another prospective UK study showed that obese or overweight children received paracetamol at greater than formulary dosing standards compared with healthy children, without adequate adjustment according to ideal body weight or lean body mass, which may result in drug toxicity [23]. As concluded from recently published literature analysis findings, data supporting recommendations for paracetamol dosing strategies for pain and fever in obese and overweight children are limited, placing such children at increased risk for adverse outcomes or suboptimal efficacy [24]. Overall, the available information on the pharmacokinetics of paracetamol in children suggests that weight-based dosing should provide acceptable efficacy and safety.
3.2 Ibuprofen
Ibuprofen is rapidly absorbed from the stomach, with a Cmax of 35.8 mg/L, tmax of 0.74 h, and t½ of 6 h in children. Its antipyretic effect generally lasts approximately 6 h [25], and time to onset of the antipyretic effect is shortest for the liquid formulation compared with tablets [26]. The influence of age and weight on the pharmacokinetics of ibuprofen has not been thoroughly studied; however, the results of maturation modeling investigations demonstrated that the clearance of ibuprofen in term infants was 90% of adult levels by 1 month of age and 98% of adult levels by 3 months of age, with a maturation half-life of 36.8 weeks [27].
In younger infants at risk for dehydration, the use of ibuprofen has been associated with acute kidney injury [26, 28]. Overdose in children is rare [26]. Consequently, the limited information on the pharmacokinetics of ibuprofen in children suggests that it is effective and safe in most clinical situations, with specific exceptions identified.
4 Dosing Approaches
4.1 Paracetamol
Multiple formulations of paracetamol are available for use in children (e.g., liquids, solutions, syrups, suspensions, tablets, suppositories, and intravenous formulations) [29,30,31,32,33,34,35,36,37]. However, as this review describes the use of paracetamol for self-care, intravenous formulations have not been discussed. Formulations are typically selected according to each patient’s specific situation to provide the optimum delivery of medication. Most studies [23, 29, 30, 32, 36, 38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] and guidelines [26, 56,57,58,59] support administration of oral paracetamol at a dose of 15 mg/kg in pediatric patients. A Polish guideline on fever management in children noted, “The dosage range was confirmed by a systematic review of clinical studies, which showed that, compared to 10 mg/kg, a dose of 15 mg/kg seems to maintain a lower temperature for a longer time and is more effective in reducing the average temperature compared to baseline values (1.6°C vs. 1.2°C)” [26, 60]. A Japanese guideline for the management of acute otitis media also recommended a range of 10–15 mg/kg oral paracetamol [58].
The 2013 WHO Pocket Book of Hospital Care for Children recommends use of weight-based dosing of paracetamol, citing an optimal dose of 10–15 mg/kg for mild pain and fever in infants and children ≥ 2 months of age [8]. The maximum daily dosage is 60 mg/kg/day, with administration every 4 h [26]. Nearly all clinical studies [23, 29, 30, 32, 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55, 61,62,63,64,65,66,67,68,69,70] and guidelines [26, 56,57,58,59] captured in the literature search clearly followed weight-based dosing for paracetamol.
4.2 Ibuprofen
Formulations of ibuprofen used in children include tablets, syrups, suspensions, and intravenous formulations [30, 33, 34, 40]. However, as this review describes the use of ibuprofen for self-care, intravenous formulations have not been discussed. Clinical studies [30, 40, 45,46,47, 49, 50, 53, 54, 63,64,65,66, 71,72,73] and guidelines [56, 57, 59] support weight-based administration for oral ibuprofen in children at a dose of 5–10 mg/kg, with the Polish guideline on fever management recommending a range of 5–10 mg/kg and a maximum daily dosage of 30 mg/kg/day (in children up to 35 kg) or 400 mg every 6 h (in children > 40 kg) [26]. The 2013 WHO Pocket Book of Hospital Care for Children recommends use of weight-based dosing of ibuprofen, citing an optimal dose of 5–10 mg/kg for mild pain and 10 mg/kg for fever [8].
Overall, the results of the literature search support weight-based dosing for both paracetamol and ibuprofen in children. A summary of the dosing guidelines retrieved is shown in Table 3.
5 Indications
5.1 Paracetamol
Paracetamol is the first-line treatment for pain and fever in pediatric patients worldwide and the only recommended analgesic for infants between 1 and 3 months of age [8]. Numerous studies were identified that investigated the use of paracetamol in children for a variety of indications, as described below.
The antipyretic effects of paracetamol have been well established in randomized clinical trials [29, 30, 41,42,43, 49,50,51,52,53, 62, 63, 65, 66, 74, 75]. Treatment with paracetamol reduced dental or tooth extraction pain; > 75% of patients did not require a second dose of analgesic after the first dose of paracetamol at 15 mg/kg [38]. The reported effects of prophylactic administration on post-extraction pain have not been consistent [33, 34, 39, 44, 68, 76]. Findings of representative clinical trials are summarized in Table 2.
A network meta-analysis of randomized controlled trials demonstrated that paracetamol is an effective treatment for migraine in pediatric patients [77]. Guidelines from the American Academy of Neurology (AAN) and the French Society for the Study of Migraine Headache mention paracetamol for the treatment of migraine in children [78, 79]. A blinded, randomized, controlled head-to-head study of paracetamol and ibuprofen in children with migraine found that both drugs achieved similar rates of pain freedom and pain relief, with no differences in tolerability [40].
One randomized, double-blind, placebo-controlled study assessed the effect of a single dose of paracetamol syrup in children 6–12 years of age with pharyngotonsillitis (sore throat) [37]. Patients were randomized 1:1:1 to receive paracetamol syrup 12 mg/kg, open-label ketoprofen lysine salt 40 mg, or double-blind placebo. Greater improvements in sore throat pain over time were observed with paracetamol versus placebo, and improvements with paracetamol were similar to those with ketoprofen lysine salt [37]. Additionally, results from three randomized clinical trials demonstrated the efficacy and tolerability of paracetamol for treating preoperative and/or postoperative tonsillectomy pain [45,46,47].
Administration of paracetamol as prophylaxis or treatment for vaccine complications has been investigated in several studies [36, 48, 54, 55, 69, 70, 80,81,82,83,84]. Administration of paracetamol following routine vaccinations in children 6 weeks to 9 months of age reduced the incidence of post-vaccination fever compared with placebo [85]. Paracetamol prophylaxis is not recommended for vaccine complications.
Effects of analgesic treatment have been investigated to confirm a lack of interference with vaccine immunogenicity. A study of children 6–47 months of age who received paracetamol to ameliorate fever after administration of an inactivated influenza vaccine showed no evidence of a blunted immune response [81]. Furthermore, a post hoc analysis of a randomized, multicenter study in infants 6–8 weeks of age showed that paracetamol did not affect the immune response to a diphtheria, tetanus, pertussis, hepatitis B, and Haemophilus influenzae type B combination vaccine, regardless of paracetamol administration timing [86].
5.2 Ibuprofen
Use of ibuprofen for infants < 3 months of age is not recommended by the WHO, which includes paracetamol as the only recommended analgesic for this population [8]. However, recent studies that directly compared ibuprofen and paracetamol in infants < 3 months of age demonstrated benefits of ibuprofen over paracetamol (e.g., reduced temperature, less pain) [28, 30, 31]. Some treatment guidelines state that both drugs have comparable efficacy but recommend paracetamol over ibuprofen in specific conditions, such as dehydration or risk for dehydration, which often accompanies fever, and in patients with varicella, pneumonia, Kawasaki disease, or coagulation disorders, because of an increased toxicity of ibuprofen compared with paracetamol [56].
Studies were identified that investigated the use of ibuprofen in children for several indications including fever [28, 30, 42, 43, 49, 50, 53, 63,64,65,66, 71, 87], dental or tooth extraction pain [33, 34, 44, 76], and migraine [40, 73, 77]. Findings of representative studies are summarized in Table 2. Ibuprofen is also recommended for the treatment of migraine in children in the guidelines of the AAN and the French Society for the Study of Migraine Headache [78, 79].
One study in children with uncomplicated extremity fractures compared the analgesic effect of ibuprofen with that of oral morphine [72]. While both drugs improved pain scores 30 min after administration, treatment with morphine was associated with significantly more adverse effects than treatment with ibuprofen (p < 0.01) [72]. The efficacy and tolerability of ibuprofen in the treatment of preoperative and/or postoperative tonsillectomy pain were demonstrated in randomized clinical trials in which paracetamol was a comparator (Table 2) [45,46,47].
In children immunized with an inactivated influenza vaccine, administration of ibuprofen following vaccination to alleviate fever did not result in a blunted immune response [81]. Prophylactic administration of ibuprofen did not appear to interfere with the immune response to vaccination with pneumococcal conjugate vaccines [36, 88], but it did interfere with the immune response to diphtheria-tetanus-pertussis vaccine (DTaP)/human papilloma virus (HBV)/inactivated polio vaccine (IPV)/Haemophilus influenzae type B vaccine (Hib) in a randomized, controlled trial [88]. Lack of interference with the immune response to the pneumococcal conjugated vaccine (Hemophilus influenzae protein D-conjugate vaccine [PHiD-CV]) was observed regardless of the timing of prophylactic ibuprofen administration (immediate or delayed during primary or booster vaccination) [89].
6 Paracetamol and Ibuprofen for COVID-19 Symptoms and Post–COVID-19 Immunization Symptoms in Children
As of May 2022, the number of confirmed COVID-19 infections reported by the WHO was over 500 million globally, and rates continue to rise [90]. COVID-19, caused by the severe acute respiratory syndrome coronavirus 2, is associated with a wide array of symptoms, including a spectrum of pulmonary symptoms such as dyspnea (with or without chronic oxygen dependence), fibrotic lung damage, and challenges in discontinuing ventilator use [91, 92]. Patients with COVID-19 infection also display an array of other symptoms. In one outpatient study of patients with COVID-19 infection as defined by WHO criteria (N = 1487), the most common symptoms reported were fever and cough (91%), asthenia (60%), body aches/myalgia (57%), headache (55%), dyspnea (32%), chest pain (22%), and ear/nose/throat symptoms such as anosmia (28%), ageusia (28%), and ageusia + anosmia (23%) [93].
The NIH COVID-19 treatment guidelines recommend supportive care for the management of children infected with COVID-19 and for the subset of children who develop multisystem inflammatory syndrome (MIS-C), but they do not specify drugs or regimens. The treatment guidelines also note that individuals infected with COVID-19 who are receiving NSAIDs for an underlying medical condition should not discontinue NSAID therapy unless warranted and that use of paracetamol and NSAIDs for antipyretic therapy should remain similar to that for other patients [94]. The WHO Living Guidance for Clinical Management of COVID-19 (23 November 2021 version) [95] includes a conditional recommendation for the use of corticosteroids in addition to high-quality supportive care for children with MIS-C, referring clinicians to the 2013 WHO Pocket Book of Hospital Care for Children [8] for guidance on supportive care management. Consequently, the role of paracetamol and ibuprofen for management of COVID-19 symptoms is not well defined.
COVID-19 is among the top 10 causes of death for children 5–11 years of age, and as of April 15, 2022, the CDC recommends that all individuals ≥ 6 months of age receive a COVID-19 vaccine [96]. The Pfizer-BioNTech mRNA vaccine (COMIRNATY) has received full US Food and Drug Administration approval for use in individuals ≥ 16 years of age [97] and individuals 12–15 years of age, and has emergency use authorization for children 5–11 years of age. The Pfizer-BioNTech vaccine has also received approval from the European Commission for use in individuals ≥ 12 years of age [98]. The Moderna mRNA vaccine, the Johnson & Johnson/Janssen adenoviral vector vaccine, and the Sinovac-Coronavac inactivated virus vaccine currently have emergency use authorization only for individuals ≥ 18 years of age [99, 100].
The CDC does not recommend the use of analgesics prior to COVID-19 vaccination to prevent side effects. However, paracetamol and NSAIDs may have a role in post-vaccination symptom relief, as the CDC recommends asking the child’s healthcare provider for advice on using a non-aspirin analgesic to manage side effects such as injection site pain and swelling, muscle pain, fever, chills, and headache. One publication captured in the literature search suggests the use of paracetamol to treat these symptoms in children [101]. The findings demonstrate that the role of paracetamol and ibuprofen in the management of post–COVID-19 immunization symptoms in children is not well established and will likely continue to evolve as younger children become eligible for vaccination.
7 Discussion
Children experience a wide range of conditions associated with pain and fever. Paracetamol and ibuprofen are used globally in pediatric patients for the treatment of multiple indications involving pain and fever. Clinical studies and guidelines support weight-based dosing of oral paracetamol and ibuprofen in children, with recommended dosages of 10–15 mg/day for paracetamol (maximum of 60 mg/kg/day) and 5–10 mg/kg for ibuprofen (maximum of 30 mg/kg/day in children ≤ 35 kg or 400 mg every 6 h in children > 40 kg). However, no study in pediatric patients could be identified that investigated paracetamol treatment of muscle ache, musculoskeletal pain, or earache (acute otitis media) at the above recommended doses, and only one study investigated paracetamol treatment and ibuprofen for sore throat pain [37]. Similarly, none of the identified studies investigated ibuprofen treatment for acute otitis media pain. Given that parents of children ages 0–18 in GPI-4 most commonly identified sore throat as a pain condition experienced by their children, more studies of paracetamol and ibuprofen in children are needed for this and other pain indications.
Prophylaxis with paracetamol or ibuprofen to manage vaccine reactions is not recommended, as evidence suggests that it is either ineffective [54] or can negatively affect vaccine immunogenicity (e.g., DTaP/HBV/IPV/Hib) [36, 88, 89], depending on the specific vaccine administered. However, paracetamol or ibuprofen use as treatment for reactions to vaccines such as diphtheria, tetanus, pertussis, hepatitis B, and Hib combination does not appear to affect vaccine immunogenicity [81, 86]. Presently, the roles of paracetamol and ibuprofen in COVID-19 infection symptom management and post–COVID-19 vaccination symptom management are not well defined in children and will continue to evolve.
Limitations of this report are typical of those associated with narrative literature reviews and include the subjective nature of the narrative review process and the potential for selection bias of the literature discussed. Although these limitations can be addressed by conducting a systematic literature review, this method was not feasible because of the broad scope of this review. However, we believe that the limitations noted were minimized by following a predefined set of inclusion and exclusion criteria for article selection, as outlined in the “Methods.”
8 Conclusion
Accurate, personalized dosing of paracetamol and ibuprofen for selfcare indications is needed for optimal symptom management, as age, weight, and pharmacokinetics can influence dosing approaches in children. Well-designed studies of these medications for the treatment of pain and fever associated with COVID-19 in pediatric patients are urgently needed to ensure optimal relief with minimal toxicity.
References
Hagen M, Madhavan T, Bell J. Combined analysis of 3 cross-sectional surveys of pain in 14 countries in Europe, the Americas, Australia, and Asia: impact on physical and emotional aspects and quality of life. Scand J Pain. 2020;20(3):575–89. https://doi.org/10.1515/sjpain-2020-0003.
Stevens BJ, Zempsky WT. Prevalence and distribution of pain in children. In: Stevens BJ, Hathway G, Zempsky WT, editors. Oxford textbook of pediatric pain. 2nd ed. Oxford: Oxford University Press; 2021.
Global Pain Index Report: GSK Consumer Healthcare; 2020. https://www.gsk.com/media/6351/2020-global-plain-index-report.pdf.
de Leeuw TG, Dirckx M, Gonzalez Candel A, Scoones GP, Huygen F, de Wildt SN. The use of dipyrone (metamizol) as an analgesic in children: What is the evidence? A review. Paediatr Anaesth. 2017;27(12):1193–201. https://doi.org/10.1111/pan.13257.
Ntamabyaliro NY, Burri C, Nzolo DB, Engo AB, Lula YN, Mampunza SM, Nsibu CN, Mesia GK, Kayembe JN, Likwela JL, Kintaudi LM, Tona GL. Drug use in the management of uncomplicated malaria in public health facilities in the Democratic Republic of the Congo. Malar J. 2018;17(1):189. https://doi.org/10.1186/s12936-018-2332-3.
Ohashi N, Kohno T. Analgesic effect of acetaminophen: a review of known and novel mechanisms of action. Front Pharmacol. 2020;11:580289. https://doi.org/10.3389/fphar.2020.580289.
Przybyła GW, Szychowski KA, Gmiński J. Paracetamol – An old drug with new mechanisms of action. Clin Exp Pharmacol Physiol. 2021;48(1):3–19. https://doi.org/10.1111/1440-1681.13392.
World Health Organization. Pocket book of hospital care for children. In: Guidelines for the management of common childhood illnesses. 2nd ed. Geneva, Switzerland: World Health Organization; 2013.
van Rensburg R, Reuter H. An overview of analgesics: NSAIDs, paracetamol, and topical analgesics Part 1. S Afr Fam Prac. 2019;61(Suppl 1):S4–10. https://doi.org/10.1080/20786190.2019.1610228.
de Martino M, Chiarugi A. Recent advances in pediatric use of oral paracetamol in fever and pain management. Pain Ther. 2015;4(2):149–68. https://doi.org/10.1007/s40122-015-0040-z.
Hopkins CS, Underhill S, Booker PD. Pharmacokinetics of paracetamol after cardiac surgery. Arch Dis Child. 1990;65(9):971–6. https://doi.org/10.1136/adc.65.9.971.
Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291s-s298.
Li J, Chiew AL, Isbister GK, Duffull SB. Sulfate conjugation may be the key to hepatotoxicity in paracetamol overdose. Br J Clin Pharmacol. 2021;87(5):2392–6. https://doi.org/10.1111/bcp.14642.
Moriarty C, Carroll W. Paracetamol: pharmacology, prescribing and controversies. Arch Dis Child Educ Pract Ed. 2016;101(6):331–4. https://doi.org/10.1136/archdischild-2014-307287.
Anderson BJ, Woollard GA, Holford NH. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol. 2000;50(2):125–34.
Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NH. Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology. 2002;96(6):1336–45. https://doi.org/10.1097/00000542-200206000-00012.
Ji P, Wang Y, Li Z, Doddapaneni S, Hertz S, Furness S, Sahajwalla CG. Regulatory review of acetaminophen clinical pharmacology in young pediatric patients. J Pharm Sci. 2012;101(12):4383–9. https://doi.org/10.1002/jps.23331.
Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol. 2016;4(2):131–42. https://doi.org/10.14218/jcth.2015.00052.
Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug Metab Rev. 2011;43(1):41–52. https://doi.org/10.3109/03602532.2010.527984.
Piana C, Danhof M, Della PO. Influence of covariate distribution on the predictive performance of pharmacokinetic models in paediatric research. Br J Clin Pharmacol. 2014;78(1):145–57. https://doi.org/10.1111/bcp.12322.
Cella M, Knibbe C, Danhof M, Della PO. What is the right dose for children? Br J Clin Pharmacol. 2010;70(4):597–603. https://doi.org/10.1111/j.1365-2125.2009.03591.x.
Burton ZA, Lewis R, Bennett T, Lewis H, Heikal S, Selman A, Sinha D, McLernon DJ, Engelhardt T, Brooks P, Edwards M, Paediatric Anaesthesia Trainee Research Network. Perioperative paracetamol prescribing in obese versus normal weight children in the UK: a national prospective multicentre observational cohort study abstract. Anesth Analg. 2021;133(3(Suppl 2)):1194–5. https://doi.org/10.1213/01.ane.0000791544.44545.82.
Husain N, Wadwa A, Christiansen N. Evaluation of analgesic doses prescribed postoperatively for overweight and obese children [abstract P20]. Arch Dis Child. 2020;105(9):e16–7. https://doi.org/10.1136/archdischild-2020-NPPG.29.
Zempsky WT, Bhagat PK, Siddiqui K. Practical challenges-use of paracetamol in children and youth who are overweight or obese: a narrative review. Paediatr Drugs. 2020;22(5):525–34. https://doi.org/10.1007/s40272-020-00417-z.
Kauffman RE, Nelson MV. Effect of age on ibuprofen pharmacokinetics and antipyretic response. J Pediatr. 1992;121(6):969–73. https://doi.org/10.1016/s0022-3476(05)80354-3.
Doniec Z, Jackowska T, Sybilski A, Woroń J, Mastalerz-Migas A. FEVER in children – recommendations for primary care doctors—FEVER COMPASS. Fam Med Prim Care Rev. 2021;23(1):99–115. https://doi.org/10.5114/fmpcr.2021.102648.
Anderson BJ, Hannam JA. A target concentration strategy to determine ibuprofen dosing in children. Paediatr Anaesth. 2019;29(11):1107–13. https://doi.org/10.1111/pan.13731.
Tan E, Braithwaite I, McKinlay CJD, Dalziel SR. Comparison of acetaminophen (paracetamol) with ibuprofen for treatment of fever or pain in children younger than 2 years: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(10):e2022398. https://doi.org/10.1001/jamanetworkopen.2020.22398.
Okereke B, Ibeleme O, Bisi-Onyemaechi A. Randomised comparative trial of the efficacy of paracetamol syrup and dispersible tablets for the treatment of fever in children. J Int Med Res. 2021;49(3):300060521999755. https://doi.org/10.1177/0300060521999755.
Alaje EO, Udoh EE, Akande PA, Odey FA, Meremikwu MM. Ibuprofen versus paracetamol for treating fever in preschool children in Nigeria: a randomized clinical trial of effectiveness and safety. Pan Afr Med J. 2020;36:350. https://doi.org/10.11604/pamj.2020.36.350.21393.
Paul IM, Walson PD. Acetaminophen and ibuprofen in the treatment of pediatric fever: a narrative review. Curr Med Res Opin. 2021;37(8):1363–75. https://doi.org/10.1080/03007995.2021.1928617.
Poonai N, Kumar K, Coriolano K, Thompson G, Brahmbhatt S, Dzongowski E, Stevens H, Gupta P, Miller M, Elsie S, Ashok D, Joubert G, Lim R, Bütter A, Ali S. Hyoscine butylbromide versus acetaminophen for nonspecific colicky abdominal pain in children: a randomized controlled trial. CMAJ. 2020;192(48):e1612–9. https://doi.org/10.1503/cmaj.201055.
Raslan N, Zouzou T. Comparison of preemptive ibuprofen, acetaminophen, and placebo administration in reducing peri- and postoperative pain in primary tooth extraction: a randomized clinical trial. Clin Exp Dent Res. 2021;7(6):1045–52. https://doi.org/10.1002/cre2.465.
Santos PS, Massignan C, de Oliveira EV, Miranda Santana C, Bolan M, Cardoso M. Does the pre-emptive administration of paracetamol or ibuprofen reduce trans- and post-operative pain in primary molar extraction? A randomized placebo-controlled clinical trial. Int J Paediatr Dent. 2020;30(6):782–90. https://doi.org/10.1111/ipd.12649.
van Uum RT, Venekamp RP, Zuithoff NP, Sjoukes A, van de Pol AC, Schilder AG, Damoiseaux RA. Improving pain management in childhood acute otitis media in general practice: a cluster randomised controlled trial of a GP-targeted educational intervention. Br J Gen Pract. 2020;70(699):e684–95. https://doi.org/10.3399/bjgp20X712589.
Koufoglou E, Kourlaba G, Michos A. Effect of prophylactic administration of antipyretics on the immune response to pneumococcal conjugate vaccines in children: a systematic review. Pneumonia (Nathan Qld). 2021;13(1):7. https://doi.org/10.1186/s41479-021-00085-8.
Ruperto N, Carozzino L, Jamone R, Freschi F, Picollo G, Zera M, Della Casa Alberighi O, Salvatori E, Del Vecchio A, Dionisio P, Martini A. A randomized, double-blind, placebo-controlled trial of paracetamol and ketoprofren lysine salt for pain control in children with pharyngotonsillitis cared by family pediatricians. Ital J Pediatr. 2011;37:48. https://doi.org/10.1186/1824-7288-37-48.
Baillargeau C, Lopez-Cazaux S, Charles H, Ordureau A, Dajean-Trutaud S, Prud’homme T, Hyon I, Soueidan A, Alliot-Licht B, Renard E. Post-operative discomforts in children after extraction of primary teeth. Clin Exp Dent Res. 2020;6(6):650–8. https://doi.org/10.1002/cre2.316.
Fux-Noy A, Bendahan Y, Ungar E, Shmueli A, Halperson E, Ram D, Moskovitz M. Does preoperative paracetamol reduce pain after dental treatment? A randomized controlled double-blind study. Quintessence Int. 2020;51(9):732–40. https://doi.org/10.3290/j.qi.a45101.
Pavithra V, Mishra D, Behera S, Juneja M. Paracetamol versus ibuprofen for the acute treatment of migraine headache in children: a blinded randomized controlled trial. Indian J Pediatr. 2020;87(10):781–6. https://doi.org/10.1007/s12098-020-03315-x.
Senel S, Erkek N, Karacan CD. Comparison of acetaminophen and ketoprofen in febrile children: a single dose randomized clinical trial. Indian J Pediatr. 2012;79(2):213–7. https://doi.org/10.1007/s12098-011-0500-3.
Kokki H, Kokki M. Ketoprofen versus paracetamol (acetaminophen) or ibuprofen in the management of fever: results of two randomized, double-blind, double-dummy, parallel-group, repeated-dose, multicentre, phase III studies in children. Clin Drug Investig. 2010;30(6):375–86. https://doi.org/10.2165/11534930-000000000-00000.
Jayawardena S, Kellstein D. Antipyretic efficacy and safety of ibuprofen versus acetaminophen suspension in febrile children: results of 2 randomized, double-blind, single-dose studies. Clin Pediatr (Phila). 2017;56(12):1120–7.
Kharouba J, Ratson T, Somri M, Blumer S. Preemptive analgesia by paracetamol, ibuprofen or placebo in pediatric dental care: a randomized controlled study. J Clin Pediatr Dent. 2019;43(1):51–5. https://doi.org/10.17796/1053-4625-43.1.10.
Mahgoobifard M, Mirmesdagh Y, Imani F, Najafi A, Nataj-Majd M. The analgesic efficacy of preoperative oral Ibuprofen and acetaminophen in children undergoing adenotonsillectomy: a randomized clinical trial. Anesth Pain Med. 2014;4(1):e15049. https://doi.org/10.5812/aapm.15049.
Mirashrafi F, Tavakolnejad F, Amirzargar B, Abasi A, Amali A. Effect of paracetamol versus ibuprofen in adenotonsillectomy. Iran J Otorhinolaryngol. 2021;33(119):355–9. https://doi.org/10.22038/ijorl.2021.56501.2945.
Jotić A, Savić Vujović K, Milovanović J, Vujović A, Radin Z, Milić N, Vučković S, Medić B, Prostran M. Pain management after surgical tonsillectomy: is there a favorable analgesic? Ear Nose Throat J. 2019;98(6):356–61. https://doi.org/10.1177/0145561319846065.
Karambin MM, Heidarzadeh A, Sharghy R, Dalili S, Hashemian H. Effects of administering prophylactic acetaminophen on short-term complications of vaccination in 6-month-old infants. Int J Prev Med. 2015;6:124. https://doi.org/10.4103/2008-7802.172380.
Paul IM, Sturgis SA, Yang C, Engle L, Watts H, Berlin CM Jr. Efficacy of standard doses of ibuprofen alone, alternating, and combined with acetaminophen for the treatment of febrile children. Clin Ther. 2010;32(14):2433–40. https://doi.org/10.1016/j.clinthera.2011.01.006.
Kramer LC, Richards PA, Thompson AM, Harper DP, Fairchok MP. Alternating antipyretics: antipyretic efficacy of acetaminophen versus acetaminophen alternated with ibuprofen in children. Clin Pediatr (Phila). 2008;47(9):907–11. https://doi.org/10.1177/0009922808319967.
Hay AD, Costelloe C, Redmond NM, Montgomery AA, Fletcher M, Hollinghurst S, Peters TJ. Paracetamol plus ibuprofen for the treatment of fever in children (PITCH): randomised controlled trial. BMJ. 2008;337:a1302. https://doi.org/10.1136/bmj.a1302.
Friedman AD, Barton LL. Efficacy of sponging vs acetaminophen for reduction of fever. Sponging Study Group. Pediatr Emerg Care. 1990;6(1):6–7. https://doi.org/10.1097/00006565-199003000-00003.
Walson PD. Ibuprofen versus paracetamol for the treatment of fever in children. Br J Clin Pract Suppl. 1990;70:19–21.
Jackson LA, Dunstan M, Starkovich P, Dunn J, Yu O, Nelson JC, Rees T, Zavitkovsky A. Prophylaxis with acetaminophen or ibuprofen for prevention of local reactions to the fifth diphtheria-tetanus toxoids-acellular pertussis vaccination: a randomized, controlled trial. Pediatrics. 2006;117(3):620–5. https://doi.org/10.1542/peds.2005-1217.
Ipp MM, Gold R, Greenberg S, Goldbach M, Kupfert BB, Lloyd DD, Maresky DC, Saunders N, Wise SA. Acetaminophen prophylaxis of adverse reactions following vaccination of infants with diphtheria-pertussis-tetanus toxoids-polio vaccine. Pediatr Infect Dis J. 1987;6(8):721–5. https://doi.org/10.1097/00006454-198708000-00005.
Doria M, Careddu D, Iorio R, Verrotti A, Chiappini E, Barbero GM, Ceschin F, Dell’Era L, Fabiano V, Mencacci M, Carlomagno F, Libranti M, Mazzone T, Vitale A. Paracetamol and ibuprofen in the treatment of fever and acute mild-moderate pain in children: Italian experts’ consensus statements. Children (Basel). 2021. https://doi.org/10.3390/children8100873.
Green R, Webb D, Jeena PM, Wells M, Butt N, Hangoma JM, Moodley RS, Maimin J, Wibbelink M, Mustafa F. Management of acute fever in children: consensus recommendations for community and primary healthcare providers in sub-Saharan Africa. Afr J Emerg Med. 2021;11(2):283–96. https://doi.org/10.1016/j.afjem.2020.11.004.
Hayashi T, Kitamura K, Hashimoto S, Hotomi M, Kojima H, Kudo F, Maruyama Y, Sawada S, Taiji H, Takahashi G, Takahashi H, Uno Y, Yano H. Clinical practice guidelines for the diagnosis and management of acute otitis media in children-2018 update. Auris Nasus Larynx. 2020;47(4):493–526. https://doi.org/10.1016/j.anl.2020.05.019.
Sullivan JE, Farrar HC. Fever and antipyretic use in children. Pediatrics. 2011;127(3):580–7. https://doi.org/10.1542/peds.2010-3852.
Temple AR, Temple BR, Kuffner EK. Dosing and antipyretic efficacy of oral acetaminophen in children. Clin Ther. 2013;35(9):1361-75.e1-45. https://doi.org/10.1016/j.clinthera.2013.06.022.
Motov S, Butt M, Masoudi A, Palacios W, Fassassi C, Drapkin J, Likourezos A, Hossain R, Brady J, Rothberger N, Flom P, Zerzan J, Marshall J. Comparison of oral ibuprofen and acetaminophen with either analgesic alone for pediatric emergency department patients with acute pain. J Emerg Med. 2020;58(5):725–32. https://doi.org/10.1016/j.jemermed.2020.02.010.
Thomas S, Vijaykumar C, Naik R, Moses PD, Antonisamy B. Comparative effectiveness of tepid sponging and antipyretic drug versus only antipyretic drug in the management of fever among children: a randomized controlled trial. Indian Pediatr. 2009;46(2):133–6.
Wong A, Sibbald A, Ferrero F, Plager M, Santolaya ME, Escobar AM, Campos S, Barragan S, De Leon GM, Kesselring GL. Antipyretic effects of dipyrone versus ibuprofen versus acetaminophen in children: results of a multinational, randomized, modified double-blind study. Clin Pediatr (Phila). 2001;40(6):313–24. https://doi.org/10.1177/000992280104000602.
Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics. 1999;104(4):e39.
Vauzelle-Kervroedan F, d’Athis P, Pariente-Khayat A, Debregeas S, Olive G, Pons G. Equivalent antipyretic activity of ibuprofen and paracetamol in febrile children. J Pediatr. 1997;131(5):683–7.
Sidler J, Frey B, Baerlocher K. A double-blind comparison of ibuprofen and paracetamol in juvenile pyrexia. Br J Clin Pract Suppl. 1990;70:22–5.
Thompson J, Fleet W, Lawrence E, Pierce E, Morris L, Wright P. A comparison of acetaminophen and rimantadine in the treatment of influenza A infection in children. J Med Virol. 1987;21(3):249–55. https://doi.org/10.1002/jmv.1890210308.
O’Donnell A, Henderson M, Fearne J, O’Donnell D. Management of postoperative pain in children following extractions of primary teeth under general anaesthesia: a comparison of paracetamol, Voltarol and no analgesia. Int J Paediatr Dent. 2007;17(2):110–5. https://doi.org/10.1111/j.1365-263X.2006.00800.x.
Uhari M, Hietala J, Viljanen MK. Effect of prophylactic acetaminophen administration on reaction to DTP vaccination. Acta Paediatr Scand. 1988;77(5):747–51. https://doi.org/10.1111/j.1651-2227.1988.tb10741.x.
Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, Smetana J, Lommel P, Kaliskova E, Borys D, Schuerman L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009;374(9698):1339–50. https://doi.org/10.1016/s0140-6736(09)61208-3.
Magni AM, Scheffer DK, Bruniera P. Antipyretic effect of ibuprofen and dipyrone in febrile children. J Pediatr (Rio J). 2011;87(1):36–42. https://doi.org/10.2223/jped.2060.
Poonai N, Bhullar G, Lin K, Papini A, Mainprize D, Howard J, Teefy J, Bale M, Langford C, Lim R, Stitt L, Rieder MJ, Ali S. Oral administration of morphine versus ibuprofen to manage postfracture pain in children: a randomized trial. CMAJ. 2014;186(18):1358–63. https://doi.org/10.1503/cmaj.140907.
Dooley JM, Gordon KE, Wood EP, Brna PM, MacSween J, Fraser A. Caffeine as an adjuvant to ibuprofen in treating childhood headaches. Pediatr Neurol. 2007;37(1):42–6. https://doi.org/10.1016/j.pediatrneurol.2007.02.016.
Kinmonth AL, Fulton Y, Campbell MJ. Management of feverish children at home. BMJ. 1992;305(6862):1134–6. https://doi.org/10.1136/bmj.305.6862.1134.
Kramer MS, Naimark LE, Roberts-Bräuer R, McDougall A, Leduc DG. Risks and benefits of paracetamol antipyresis in young children with fever of presumed viral origin. Lancet. 1991;337(8741):591–4. https://doi.org/10.1016/0140-6736(91)91648-e.
Baygin O, Tuzuner T, Isik B, Kusgoz A, Tanriver M. Comparison of pre-emptive ibuprofen, paracetamol, and placebo administration in reducing post-operative pain in primary tooth extraction. Int J Paediatr Dent. 2011;21(4):306–13. https://doi.org/10.1111/j.1365-263X.2011.01124.x.
Wang G, Tan T, Liu Y, Hong P. Drugs for acute attack of pediatric migraine: a network meta-analysis of randomized controlled trials. Clin Neurol Neurosurg. 2020;195:105853. https://doi.org/10.1016/j.clineuro.2020.105853.
Oskoui M, Pringsheim T, Holler-Managan Y, Potrebic S, Billinghurst L, Gloss D, Hershey AD, Licking N, Sowell M, Victorio MC, Gersz EM, Leininger E, Zanitsch H, Yonker M, Mack K. Practice guideline update summary: acute treatment of migraine in children and adolescents: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2019;93(11):487–99. https://doi.org/10.1212/wnl.0000000000008095.
Lanteri-Minet M, Valade D, Geraud G, Lucas C, Donnet A. Revised French guidelines for the diagnosis and management of migraine in adults and children. J Headache Pain. 2014;15(1):2. https://doi.org/10.1186/1129-2377-15-2.
Dolhain J, Janssens W, Dindore V, Mihalyi A. Infant vaccine co-administration: review of 18 years of experience with GSK’s hexavalent vaccine co-administered with routine childhood vaccines. Expert Rev Vaccines. 2020;19(5):419–43. https://doi.org/10.1080/14760584.2020.1758560.
Walter EB, Hornik CP, Grohskopf L, McGee CE, Todd CA, Museru OI, Harrington L, Broder KR. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35(48 Pt B):6664–71. https://doi.org/10.1016/j.vaccine.2017.10.020.
Hayat H, Khan PS, Hayat G. The effect of prophylactic paracetamol administration on adverse reactions following DTP vaccination. Eastern J Med. 2011;16:258–60.
Prymula R, Esposito S, Zuccotti GV, Xie F, Toneatto D, Kohl I, Dull PM. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I). Hum Vaccin Immunother. 2014;10(7):1993–2004. https://doi.org/10.4161/hv.28666.
Lewis K, Cherry JD, Sachs MH, Woo DB, Hamilton RC, Tarle JM, Overturf GD. The effect of prophylactic acetaminophen administration on reactions to DTP vaccination. Am J Dis Child. 1988;142(1):62–5. https://doi.org/10.1001/archpedi.1988.02150010072025.
Jackson LA, Peterson D, Dunn J, Hambidge SJ, Dunstan M, Starkovich P, Yu O, Benoit J, Dominguez-Islas CP, Carste B, Benson P, Nelson JC. A randomized placebo-controlled trial of acetaminophen for prevention of post-vaccination fever in infants. PLoS ONE. 2011;6(6):e20102. https://doi.org/10.1371/journal.pone.0020102.
Sil A, Ravi MD, Patnaik BN, Dhingra MS, Dupuy M, Gandhi DJ, Dhaded SM, Dubey AP, Kundu R, Lalwani SK, Chhatwal J, Mathew LG, Gupta M, Sharma SD, Bavdekar SB, Rout SP, Jayanth MV, D’Cor NA, Mangarule SA, Ravinuthala S, Reddy EJ. Effect of prophylactic or therapeutic administration of paracetamol on immune response to DTwP-HepB-Hib combination vaccine in Indian infants. Vaccine. 2017;35(22):2999–3006. https://doi.org/10.1016/j.vaccine.2017.03.009.
Kloeden B, Tham D, Oakley E, Cheek J. Community use of paracetamol and ibuprofen in children with fever. J Paediatr Child Health. 2021;57(10):1640–4. https://doi.org/10.1111/jpc.15580.
Wysocki J, Center KJ, Brzostek J, Majda-Stanislawska E, Szymanski H, Szenborn L, Czajka H, Hasiec B, Dziduch J, Jackowska T, Witor A, Kopińska E, Konior R, Giardina PC, Sundaraiyer V, Patterson S, Gruber WC, Scott DA, Gurtman A. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35(15):1926–35. https://doi.org/10.1016/j.vaccine.2017.02.035.
Falup-Pecurariu O, Man SC, Neamtu ML, Chicin G, Baciu G, Pitic C, Cara AC, Neculau AE, Burlea M, Brinza IL, Schnell CN, Sas V, Lupu VV, François N, Swinnen K, Borys D. Effects of prophylactic ibuprofen and paracetamol administration on the immunogenicity and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugated vaccine (PHiD-CV) co-administered with DTPa-combined vaccines in children: an open-label, randomized, controlled, non-inferiority trial. Hum Vaccin Immunother. 2017;13(3):649–60. https://doi.org/10.1080/21645515.2016.1223001.
Coronavirus disease (COVID-19) pandemic: World Health Organization; 2021 [2021]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 16 Feb 2023.
Pollard CA, Morran MP, Nestor-Kalinoski AL. The COVID-19 pandemic: a global health crisis. Physiol Genomics. 2020;52(11):549–57. https://doi.org/10.1152/physiolgenomics.00089.2020.
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15. https://doi.org/10.1038/s41591-021-01283-z.
Lapostolle F, Schneider E, Vianu I, Dollet G, Roche B, Berdah J, Michel J, Goix L, Chanzy E, Petrovic T, Adnet F. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern Emerg Med. 2020;15(5):813–7. https://doi.org/10.1007/s11739-020-02379-z.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines: National Institutes of Health; 2021 [October 22, 2021]. https://www.covid19treatmentguidelines.nih.gov/. Accessed 16 Feb 2023.
COVID-19 clinical management. Living guidance: World Health Organization; 2021 [January 25, 2021]. Available from: https://apps.who.int/iris/bitstream/handle/10665/338882/WHO-2019-nCoV-clinical-2021.1-eng.pdf?sequence=1&isAllowed=y. Accessed 16 Feb 2023.
COVID-19 vaccines for children and teens: Centers for Disease Control and Prevention; 2021 [November 23, 2021]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/children-teens.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Frecommendations%2Fadolescents.html. Accessed 16 Feb 2023.
Comirnaty [package insert]. Mainz, Germany: BioNTech Manufacturing GmbH; 2021 August 2021.
Comirnaty [summary of product characteristics]. Mainz, Germany: BioNTech Manufacturing GmbH; 2021 November 2021.
Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) Emergency Use Authorization (EUA) of the Moderna COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19): Moderna; 2022 [January 31, 2022]. https://www.fda.gov/media/144637/download. Accessed 16 Feb 2023.
Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) Emergency Use Authorization (EUA) of the Janssen COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19): Janssen; 2022 [January 31, 2022]. https://www.janssenlabels.com/emergency-use-authorization/Janssen+COVID-19+Vaccine-HCP-fact-sheet.pdf. Accessed 16 Feb 2023.
Thompson LA, Rasmussen SA. Children and COVID-19 vaccines. JAMA Pediatr. 2021;175(8):876. https://doi.org/10.1001/jamapediatrics.2021.1974.
El-Metwally A, Salminen JJ, Auvinen A, Kautiainen H, Mikkelsson M. Lower limb pain in a preadolescent population: prognosis and risk factors for chronicity–a prospective 1- and 4-year follow-up study. Pediatrics. 2005;116(3):673–81. https://doi.org/10.1542/peds.2004-1758.
Southey ER, Soares-Weiser K, Kleijnen J. Systematic review and meta-analysis of the clinical safety and tolerability of ibuprofen compared with paracetamol in paediatric pain and fever. Curr Med Res Opin. 2009;25(9):2207–22. https://doi.org/10.1185/03007990903116255.
Yin F, Liu Y, Guo H. Comparison between ibuprofen and acetaminophen in the treatment of infectious fever in children: a meta-analysis. J Healthc Eng. 2022;2022:1794258. https://doi.org/10.1155/2022/1794258.
Bird SE, Williams K, Kula K. Preoperative acetaminophen vs ibuprofen for control of pain after orthodontic separator placement. Am J Orthod Dentofacial Orthop. 2007;132(4):504–10. https://doi.org/10.1016/j.ajodo.2006.11.019.
Primosch RE, Nichols DL, Courts FJ. Comparison of preoperative ibuprofen, acetaminophen, and placebo administration on the parental report of postextraction pain in children. Pediatr Dent. 1995;17(3):187–91.
Cuvellier JC, Donnet A, Guégan-Massardier E, Nachit-Ouinekh F, Parain D, Vallée L. Treatment of primary headache in children: a multicenter hospital-based study in France. J Headache Pain. 2009;10(6):447–53. https://doi.org/10.1007/s10194-009-0158-7.
Silver S, Gano D, Gerretsen P. Acute treatment of paediatric migraine: a meta-analysis of efficacy. J Paediatr Child Health. 2008;44(1–2):3–9. https://doi.org/10.1111/j.1440-1754.2007.01206.x.
Acknowledgments
Medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and was funded by Haleon.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This literature analysis was funded by Haleon, Warren, NJ.
Conflict of Interest
William Zempsky: Consultant for Pfizer, Endo Pharmaceuticals, GlaxoSmithKline, and Vapogenix; funding received from the National Institutes of Health, the US Department of Defense, and the Mayday Fund. John Bell: Consultant for Bayer, GlaxoSmithKline, Mylan, Novartis, Pfizer, and Reckitt Benckiser; speaker for GlaxoSmithKline, Pfizer, and Reckitt Benckiser. Vanessa Maria Mossali: Nothing to disclose. Preeti Kachroo: Employee of Haleon, Singapore. Kamran Siddiqui: Employee of Haleon, Singapore.
Author Contributions
All authors developed the idea for this review article, performed the data analysis, and critically revised the work.
Data Sharing Statement
The references cited in this narrative review are publicly available.
Ethics Approval
Not applicable.
Consent (participate & publication)
Not applicable.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zempsky, W., Bell, J., Mossali, V.M. et al. Common Selfcare Indications of Pain Medications in Children. Pediatr Drugs 25, 321–341 (2023). https://doi.org/10.1007/s40272-023-00562-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00562-1