Abstract
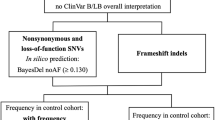
Gene sub-region encoded protein domain is the basic unit for protein structure and function. The DMD gene is the largest coding gene in humans, with its phenotype relevant to idiopathic generalized epilepsy. We hypothesized variants clustered in sub-regions of idiopathic generalized epilepsy genes and investigated the relationship between the DMD gene and idiopathic generalized epilepsy. Whole exome sequencing was performed in 106 idiopathic generalized epilepsy individuals. DMD variants were filtered with variant type, allele frequency, in silico prediction, hemizygous or homozygous status in the population, inheritance mode, and domain location. Variants located at the sub-regions were selected by the subRVIS software. The pathogenicity of variants was evaluated by the American College of Medical Genetics and Genomics criteria. Articles on functional studies related to epilepsy for variants clustered protein domains were reviewed. In sub-regions of the DMD gene, two variants were identified in two unrelated cases with juvenile absence epilepsy or juvenile myoclonic epilepsy. The pathogenicity of both variants was uncertain significance. Allele frequency of both variants in probands with idiopathic generalized epilepsy reached statistical significance compared with the population (Fisher’s test, p = 2.02 × 10−6, adjusted α = 4.52 × 10−6). The variants clustered in the spectrin domain of dystrophin, which binds to glycoprotein complexes and indirectly affects ion channels contributing to epileptogenesis. Gene sub-region analysis suggests a weak association between the DMD gene and idiopathic generalized epilepsy. Functional analysis of gene sub-region helps infer the pathogenesis of idiopathic generalized epilepsy.




Similar content being viewed by others
Data Availability
We state that the data is available for all readers.
References
Koonin EV, Wolf YI, Karev GP (2002) The structure of the protein universe and genome evolution. Nature 420(14):218–223
Wheelan SJ, Marchler-Bauer A, Bryant SH (2000) Domain size distributions can predict domain boundaries. Bioinformatics 16(7):613–618
Zhou H, Yang Y, Shen H-B (2017) Hum-mPLoc 3.0: prediction enhancement of human protein subcellular localization through modeling the hidden correlations of gene ontology and functional domain features. Bioinformatics 33(6):843–853
Marcotte EM, Pellegrini M, Ng H-L et al (1999) Detecting protein function and protein-protein interactions from genome sequences. Science 285(5428):751–753
Anderson JL, Head SI, Rae C et al (2002) Brain function in Duchenne muscular dystrophy. Brain 125:4–13
Hendriksen RGF, Hoogland G, Schipper S et al (2015) A possible role of dystrophin in neuronal excitability: a review of the current literature. Neurosci Biobehav R 51:255–262
Goodwin F, Muntoni F, Dubowitz V (1997) Epilepsy in Duchenne and Becker muscular dystrophies. Eur J Paediatr Neuro 1(4):115–119
Pane M, Messina S, Bruno C et al (2013) Duchenne muscular dystrophy and epilepsy. Neuromuscul Disord 23(4):313–315
Jallon P, Latour P (2005) Epidemiology of idiopathic generalized epilepsies. Epilepsia 46(Suppl 9):10–14
Kovel CGFd, Trucks H, Helbig I et al (2010) Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain. 133(1):23–32
Healy L, Moran M, Singhal S et al (2018) Relapse after treatment withdrawal of antiepileptic drugs for Juvenile Absence Epilepsy and Juvenile Myoclonic Epilepsy. Seizure 59:116–122
Peljto AL, Barker-Cummings C, Vasoli VM et al (2014) Familial risk of epilepsy: a population-based study. Brain 137(3):795–805
Vadlamudi L, Milne RL, Lawrence K et al (2014) Genetics of epilepsy: the testimony of twins in the molecular era. Neurology 83(12):1042–1048
Li M, Maljevic S, Phillips AM et al (2018) Gain-of-function HCN2 variants in genetic epilepsy. Hum Mutat 39(2):202–209
Bailey JN, de Nijs L, Bai D et al (2018) Variant intestinal-cell kinase in juvenile myoclonic epilepsy. N Engl J Med 378(11):1018–1028
Li X, Poschmann S, Chen Q et al (2018) De novo BK channel variant causes epilepsy by affecting voltage gating but not Ca2+ sensitivity. Eur J Hum Genet 26(2):220–229
Greenberg DA, Stewart WL (2014) Remind me again what disease we are studying? A population genetics, genetic analysis, and real data perspective on why progress on identifying genetic influences on common epilepsies has been so slow. Prog Brain Res 213:199–221
Gussow AB, Petrovski S, Wang Q et al (2016) The intolerance to functional genetic variation of protein domains predicts the localization of pathogenic mutations within genes. Genome Biol 17:9
Hirsch E, French J, Scheffer IE et al (2022) ILAE definition of the idiopathic generalized epilepsy syndromes: position statement by the ILAE task force on nosology and definitions. Epilepsia 63(6):1475–1499
Shi Y-W, Zhang Q, Cai K et al (2019) Synaptic clustering differences due to different GABRB3 mutations cause variable epilepsy syndromes. Brain 142(10):3028–3044
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424
Epi4K C, Epilepsy Phenome/Genome P, Allen AS et al (2013) De novo mutations in epileptic encephalopathies. Nature 501(7466):217–221
De Stefano ME, Ferretti V, Mozzetta C (2022) Synaptic alterations as a neurodevelopmental trait of Duchenne muscular dystrophy. Neurobiol Dis 168:105718
Schmitz F, Drenckhahn D (1997) Dystrophin in the retina. Prog Neurobiol 53(5):547–560
Ohlendieck K, Swandulla D (2021) Complexity of skeletal muscle degeneration: multi-systems pathophysiology and organ crosstalk in dystrophinopathy. Pflug Arch Eur J Phy 473(12):1813–1839
Percival JM (2018) Perspective: spectrin-like repeats in dystrophin have unique binding preferences for syntrophin adaptors that explain the mystery of how nNOSmu localizes to the sarcolemma. Front Physiol 9:1369
Nykamp K, Anderson M, Powers M et al (2017) Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet Med 19(10):1105–1117
Paramore S, Ayton GS, Voth GA (2006) Extending a spectrin repeat unit. II: rupture behavior. Biophys J 90(1):101–111
MacArthur DG, Manolio TA, Dimmock DP et al (2014) Guidelines for investigating causality of sequence variants in human disease. Nature 508(7497):469–476
Palmer EE, Stuhlmann T, Weinert S et al (2018) De novo and inherited mutations in the X-linked gene CLCN4 are associated with syndromic intellectual disability and behavior and seizure disorders in males and females. Mol Psychiatry 23(2):222–230
Zhou P, He N, Zhang JW et al (2018) Novel mutations and phenotypes of epilepsy-associated genes in epileptic encephalopathies. Genes Brain Behav 17(8):e12456
Ma S, Blair MA, Abou-Khalil B et al (2006) Mutations in the GABRA1 and EFHC1 genes are rare in familial juvenile myoclonic epilepsy. Epilepsy Res 71(2–3):129–134
Tanaka M, Olsen RW, Medina MT et al (2008) Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am J Hum Genet 82(6):1249–1261
Larsen J, Johannesen KM, Ek J et al (2015) The role of SLC2A1 mutations in myoclonic astatic epilepsy and absence epilepsy, and the estimated frequency of GLUT1 deficiency syndrome. Epilepsia 56(12):e203–e208
Rapaport D, Passos-Bueno MR, Brando L et al (1991) Apparent association of mental retardation and specific patterns of deletions screened with probes cf56a and cf23a in Duchenne muscular dystrophy. Am J Med Genet A 39(4):437–441
Harper SQ, Hauser MA, DelloRusso C et al (2002) Modular flexibility of dystrophin_ Implications for gene therapy of Duchenne muscular dystrophy. Nat Med 8:253–261
Legardinier S, Hubert JF, Le Bihan O et al (2008) Sub-domains of the dystrophin rod domain display contrasting lipid-binding and stability properties. Biochim Biophys Acta 1784(4):672–682
Sarkis J, Hubert JF, Legrand B et al (2011) Spectrin-like repeats 11–15 of human dystrophin show adaptations to a lipidic environment. J Biol Chem 286(35):30481–30491
Zhao J, Yang HT, Wasala L et al (2019) Dystrophin R16/17 protein therapy restores sarcolemmal nNOS in trans and improves muscle perfusion and function. Mol Med 25(1):31
Harper SQ, Crawford RW, DelloRusso C et al (2002) Spectrin-like repeats from dystrophin and alpha-actinin-2 are not functionally interchangeable. Hum Mol Genet 11(16):1807–1815
Fry AE, Marra C, Derrick AV et al (2021) Missense variants in the N-terminal domain of the A isoform of FHF2/FGF13 cause an X-linked developmental and epileptic encephalopathy. Am J Hum Genet 108(1):176–185
Funding
This study is funded by the Fujian Provincial Health Technology Project (grant number 2019-ZQN-94) and the Natural Science Foundation of Fujian Province (grant number 2020J011257). The funders had no role in study design, data collection, data analysis, data interpretation, and decision to prepare or publish the manuscript.
Author information
Authors and Affiliations
Contributions
Zhi-Jian Lin contributed to the conception and design of the study. Zhi-Jian Lin and Bi-Xia Huang performed material preparation, data collection, and analysis. Zhi-Jian Lin wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Gene sub-region analysis in individuals with idiopathic generalized epilepsy.
• Variants clustered in spectrin in the idiopathic generalized epilepsy cases.
• Spectrin indirectly affects ion channels contributing to epileptogenesis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, ZJ., Huang, BX., Su, LF. et al. Sub-region analysis of DMD gene in cases with idiopathic generalized epilepsy. Neurogenetics 24, 161–169 (2023). https://doi.org/10.1007/s10048-023-00715-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-023-00715-x