Abstract
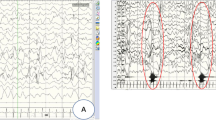
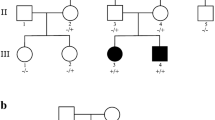
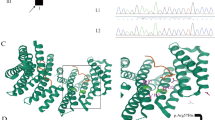
DNM1 developmental and epileptic encephalopathy (DEE) is characterized by severe to profound intellectual disability, hypotonia, movement disorder, and refractory epilepsy, typically presenting with infantile spasms. Most of the affected individuals had de novo missense variants in DNM1. DNM1 undergoes alternative splicing that results in expression of six different transcript variants. One alternatively spliced region affects the tandemly arranged exons 10a and 10b, producing isoforms DNM1A and DNM1B, respectively. Pathogenic variants in the DNM1 coding region affect all transcript variants. Recently, a de novo DNM1 NM_001288739.1:c.1197-8G > A variant located in intron 9 has been reported in several unrelated individuals with DEE that causes in-frame insertion of two amino acids and leads to disease through a dominant-negative mechanism. We report on a patient with DEE and a de novo DNM1 variant NM_001288739.2:c.1197-46C > G in intron 9, upstream of exon 10a. By RT-PCR and Sanger sequencing using fibroblast-derived cDNA of the patient, we identified aberrantly spliced DNM1 mRNAs with exon 9 spliced to the last 45 nucleotides of intron 9 followed by exon 10a (NM_001288739.2:r.1196_1197ins[1197-1_1197-45]). The encoded DNM1A mutant is predicted to contain 15 novel amino acids between Ile398 and Arg399 [NP_001275668.1:p.(Ile398_Arg399ins15)] and likely functions in a dominant-negative manner, similar to other DNM1 mutants. Our data confirm the importance of the DNM1 isoform A for normal human brain function that is underscored by previously reported predominant expression of DMN1A transcripts in pediatric brain, functional differences of the mouse Dnm1a and Dnm1b isoforms, and the Dnm1 fitful mouse, an epilepsy mouse model.


Similar content being viewed by others
References
Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O’Toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camilli P (2007) A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 316(5824):570–574. https://doi.org/10.1126/science.1140621
Robinson PJ, Sontag JM, Liu JP, Fykse EM, Slaughter C, McMahon H, Sudhof TC (1993) Dynamin GTPase regulated by protein kinase C phosphorylation in nerve terminals. Nature 365(6442):163–166. https://doi.org/10.1038/365163a0
van der Bliek AM, Meyerowitz EM (1991) Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351(6325):411–414. https://doi.org/10.1038/351411a0
Nakata T, Iwamoto A, Noda Y, Takemura R, Yoshikura H, Hirokawa N (1991) Predominant and developmentally regulated expression of dynamin in neurons. Neuron 7(3):461–469. https://doi.org/10.1016/0896-6273(91)90298-e
Scaife R, Margolis RL (1990) Biochemical and immunochemical analysis of rat brain dynamin interaction with microtubules and organelles in vivo and in vitro. J Cell Biol 111(6 Pt 2):3023–3033. https://doi.org/10.1083/jcb.111.6.3023
RM Boumil VA Letts MC Roberts C Lenz CL Mahaffey ZW Zhang T Moser WN Frankel 2010 A missense mutation in a highly conserved alternate exon of dynamin 1 causes epilepsy in fitful mice PLoS Genet 6 8. https://doi.org/10.1371/journal.pgen.1001046
Cao H, Garcia F, McNiven MA (1998) Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell 9(9):2595–2609. https://doi.org/10.1091/mbc.9.9.2595
Sontag JM, Fykse EM, Ushkaryov Y, Liu JP, Robinson PJ, Sudhof TC (1994) Differential expression and regulation of multiple dynamins. J Biol Chem 269(6):4547–4554
Urrutia R, Henley JR, Cook T, McNiven MA (1997) The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci USA 94(2):377–384. https://doi.org/10.1073/pnas.94.2.377
Antonny B, Burd C, De Camilli P, Chen E, Daumke O, Faelber K, Ford M, Frolov VA, Frost A, Hinshaw JE, Kirchhausen T, Kozlov MM, Lenz M, Low HH, McMahon H, Merrifield C, Pollard TD, Robinson PJ, Roux A, Schmid S (2016) Membrane fission by dynamin: what we know and what we need to know. EMBO J 35(21):2270–84. https://doi.org/10.15252/embj.201694613
Ferguson SM, De Camilli P (2012) Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13(2):75–88. https://doi.org/10.1038/nrm3266
Euro E-RESC, Phenome E, Genome P, Epi KC (2014) De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet 95(4):360–370. https://doi.org/10.1016/j.ajhg.2014.08.013
John A, Ng-Cordell E, Hanna N, Brkic D, Baker K (2021) The neurodevelopmental spectrum of synaptic vesicle cycling disorders. J Neurochem 157(2):208–228. https://doi.org/10.1111/jnc.15135
Fernandez-Marmiesse A, Roca I, Diaz-Flores F, Cantarin V, Perez-Poyato MS, Fontalba A, Laranjeira F, Quintans S, Moldovan O, Felgueroso B, Rodriguez-Pedreira M, Simon R, Camacho A, Quijada P, Ibanez-Mico S, Domingno MR, Benito C, Calvo R, Perez-Cejas A, Carrasco ML, Ramos F, Couce ML, Ruiz-Falco ML, Gutierrez-Solana L, Martinez-Atienza M (2019) Rare variants in 48 genes account for 42% of cases of epilepsy with or without neurodevelopmental delay in 246 pediatric patients. Front Neurosci 13:1135. https://doi.org/10.3389/fnins.2019.01135
Investigators GPP, Smedley D, Smith KR, Martin A, Thomas EA, McDonagh EM, Cipriani V, Ellingford JM, Arno G, Tucci A, Vandrovcova J, Chan G, Williams HJ, Ratnaike T, Wei W, Stirrups K, Ibanez K, Moutsianas L, Wielscher M, Need A, Barnes MR, Vestito L, Buchanan J, Wordsworth S, Ashford S, Rehmstrom K, Li E, Fuller G, Twiss P, Spasic-Boskovic O, Halsall S, Floto RA, Poole K, Wagner A, Mehta SG, Gurnell M, Burrows N, James R, Penkett C, Dewhurst E, Graf S, Mapeta R, Kasanicki M, Haworth A, Savage H, Babcock M, Reese MG, Bale M, Baple E, Boustred C, Brittain H, de Burca A, Bleda M, Devereau A, Halai D, Haraldsdottir E, Hyder Z, Kasperaviciute D, Patch C, Polychronopoulos D, Matchan A, Sultana R, Ryten M, Tavares ALT, Tregidgo C, Turnbull C, Welland M, Wood S, Snow C, Williams E, Leigh S, Foulger RE, Daugherty LC, Niblock O, Leong IUS, Wright CF, Davies J, Crichton C, Welch J, Woods K, Abulhoul L, Aurora P, Bockenhauer D, Broomfield A, Cleary MA, Lam T, Dattani M, Footitt E, Ganesan V, Grunewald S, Compeyrot-Lacassagne S, Muntoni F, Pilkington C, Quinlivan R, Thapar N, Wallis C, Wedderburn LR, Worth A, Bueser T, Compton C, Deshpande C, Fassihi H, Haque E, Izatt L, Josifova D, Mohammed S, Robert L, Rose S, Ruddy D, Sarkany R, Say G, Shaw AC, Wolejko A, Habib B, Burns G, Hunter S, Grocock RJ, Humphray SJ, Robinson PN, Haendel M, Simpson MA, Banka S, Clayton-Smith J, Douzgou S, Hall G, Thomas HB, O’Keefe RT, Michaelides M, Moore AT, Malka S, Pontikos N, Browning AC, Straub V, Gorman GS, Horvath R, Quinton R, Schaefer AM, Yu-Wai-Man P, Turnbull DM, McFarland R, Taylor RW, O’Connor E, Yip J, Newland K, Morris HR, Polke J, Wood NW, Campbell C, Camps C, Gibson K, Koelling N, Lester T, Nemeth AH, Palles C, Patel S, Roy NBA, Sen A, Taylor J, Cacheiro P, Jacobsen JO, Seaby EG, Davison V, Chitty L, Douglas A, Naresh K, McMullan D, Ellard S, Temple IK, Mumford AD, Wilson G, Beales P, Bitner-Glindzicz M, Black G, Bradley JR, Brennan P, Burn J, Chinnery PF, Elliott P, Flinter F, Houlden H, Irving M, Newman W, Rahman S, Sayer JA, Taylor JC, Webster AR, Wilkie AOM, Ouwehand WH, Raymond FL, Chisholm J, Hill S, Bentley D, Scott RH, Fowler T, Rendon A, Caulfield M (2021) 100,000 genomes pilot on rare-disease diagnosis in health care - preliminary report. N Engl J Med 385(20):1868–1880. https://doi.org/10.1056/NEJMoa2035790
Nakashima M, Kouga T, Lourenco CM, Shiina M, Goto T, Tsurusaki Y, Miyatake S, Miyake N, Saitsu H, Ogata K, Osaka H, Matsumoto N (2016) De novo DNM1 mutations in two cases of epileptic encephalopathy. Epilepsia 57(1):e18-23. https://doi.org/10.1111/epi.13257
von Spiczak S, Helbig KL, Shinde DN, Huether R, Pendziwiat M, Lourenco C, Nunes ME, Sarco DP, Kaplan RA, Dlugos DJ, Kirsch H, Slavotinek A, Cilio MR, Cervenka MC, Cohen JS, McClellan R, Fatemi A, Yuen A, Sagawa Y, Littlejohn R, McLean SD, Hernandez-Hernandez L, Maher B, Moller RS, Palmer E, Lawson JA, Campbell CA, Joshi CN, Kolbe DL, Hollingsworth G, Neubauer BA, Muhle H, Stephani U, Scheffer IE, Pena SDJ, Sisodiya SM, Helbig I, Epi KC, Euro E-RESNWG (2017) DNM1 encephalopathy: a new disease of vesicle fission. Neurology 89(4):385–394. https://doi.org/10.1212/WNL.0000000000004152
Li H, Fang F, Xu M, Liu Z, Zhou J, Wang X, Wang X, Han T (2019) Clinical assessments and EEG analyses of encephalopathies associated with dynamin-1 mutation. Front Pharmacol 10:1454. https://doi.org/10.3389/fphar.2019.01454
Lin L, Zhang Y, Pan H, Wang J, Qi Y, Ma Y (2020) Clinical and genetic characteristics and prenatal diagnosis of patients presented GDD/ID with rare monogenic causes. Orphanet J Rare Dis 15(1):317. https://doi.org/10.1186/s13023-020-01599-y
Takata A, Nakashima M, Saitsu H, Mizuguchi T, Mitsuhashi S, Takahashi Y, Okamoto N, Osaka H, Nakamura K, Tohyama J, Haginoya K, Takeshita S, Kuki I, Okanishi T, Goto T, Sasaki M, Sakai Y, Miyake N, Miyatake S, Tsuchida N, Iwama K, Minase G, Sekiguchi F, Fujita A, Imagawa E, Koshimizu E, Uchiyama Y, Hamanaka K, Ohba C, Itai T, Aoi H, Saida K, Sakaguchi T, Den K, Takahashi R, Ikeda H, Yamaguchi T, Tsukamoto K, Yoshitomi S, Oboshi T, Imai K, Kimizu T, Kobayashi Y, Kubota M, Kashii H, Baba S, Iai M, Kira R, Hara M, Ohta M, Miyata Y, Miyata R, Takanashi JI, Matsui J, Yokochi K, Shimono M, Amamoto M, Takayama R, Hirabayashi S, Aiba K, Matsumoto H, Nabatame S, Shiihara T, Kato M, Matsumoto N (2019) Comprehensive analysis of coding variants highlights genetic complexity in developmental and epileptic encephalopathy. Nat Commun 10(1):2506. https://doi.org/10.1038/s41467-019-10482-9
Bowling KM, Thompson ML, Amaral MD, Finnila CR, Hiatt SM, Engel KL, Cochran JN, Brothers KB, East KM, Gray DE, Kelley WV, Lamb NE, Lose EJ, Rich CA, Simmons S, Whittle JS, Weaver BT, Nesmith AS, Myers RM, Barsh GS, Bebin EM, Cooper GM (2017) Genomic diagnosis for children with intellectual disability and/or developmental delay. Genome Med 9(1):43. https://doi.org/10.1186/s13073-017-0433-1
Zhu X, Padmanabhan R, Copeland B, Bridgers J, Ren Z, Kamalakaran S, O’Driscoll-Collins A, Berkovic SF, Scheffer IE, Poduri A, Mei D, Guerrini R, Lowenstein DH, Allen AS, Heinzen EL, Goldstein DB (2017) A case-control collapsing analysis identifies epilepsy genes implicated in trio sequencing studies focused on de novo mutations. PLoS Genet 13(11):e1007104. https://doi.org/10.1371/journal.pgen.1007104
Deng XL, Yin F, Zhang CL, Ma YP, He F, Wu LW, Peng J (2016) Dynamin-1-related infantile spasms: a case report and review of literature. Zhonghua Er Ke Za Zhi 54(11):856–859. https://doi.org/10.3760/cma.j.issn.0578-1310.2016.11.014
Fung CW, Kwong AK, Wong VC (2017) Gene panel analysis for nonsyndromic cryptogenic neonatal/infantile epileptic encephalopathy. Epilepsia Open 2(2):236–243. https://doi.org/10.1002/epi4.12055
Yao R, Zhou Y, Tang J, Li N, Yu T, He Y, Wang C, Wang J, Wang J (2021) Genetic diagnosis spectrum and multigenic burden of exome-level rare variants in a childhood epilepsy cohort. Front Genet 12:782419. https://doi.org/10.3389/fgene.2021.782419
Allen NM, Conroy J, Shahwan A, Lynch B, Correa RG, Pena SD, McCreary D, Magalhaes TR, Ennis S, Lynch SA, King MD (2016) Unexplained early onset epileptic encephalopathy: exome screening and phenotype expansion. Epilepsia 57(1):e12–e17. https://doi.org/10.1111/epi.13250
Epi KC, Phenome E, Genome P, Allen AS, Berkovic SF, Cossette P, Delanty N, Dlugos D, Eichler EE, Epstein MP, Glauser T, Goldstein DB, Han Y, Heinzen EL, Hitomi Y, Howell KB, Johnson MR, Kuzniecky R, Lowenstein DH, Lu YF, Madou MR, Marson AG, Mefford HC, Esmaeeli Nieh S, O’Brien TJ, Ottman R, Petrovski S, Poduri A, Ruzzo EK, Scheffer IE, Sherr EH, Yuskaitis CJ, Abou-Khalil B, Alldredge BK, Bautista JF, Berkovic SF, Boro A, Cascino GD, Consalvo D, Crumrine P, Devinsky O, Dlugos D, Epstein MP, Fiol M, Fountain NB, French J, Friedman D, Geller EB, Glauser T, Glynn S, Haut SR, Hayward J, Helmers SL, Joshi S, Kanner A, Kirsch HE, Knowlton RC, Kossoff EH, Kuperman R, Kuzniecky R, Lowenstein DH, McGuire SM, Motika PV, Novotny EJ, Ottman R, Paolicchi JM, Parent JM, Park K, Poduri A, Scheffer IE, Shellhaas RA, Sherr EH, Shih JJ, Singh R, Sirven J, Smith MC, Sullivan J, Lin Thio L, Venkat A, Vining EP, Von Allmen GK, Weisenberg JL, Widdess-Walsh P, Winawer MR (2013) De novo mutations in epileptic encephalopathies. Nature 501(7466):217–221. https://doi.org/10.1038/nature12439
Ko A, Youn SE, Kim SH, Lee JS, Kim S, Choi JR, Kim HD, Lee ST, Kang HC (2018) Targeted gene panel and genotype-phenotype correlation in children with developmental and epileptic encephalopathy. Epilepsy Res 141:48–55. https://doi.org/10.1016/j.eplepsyres.2018.02.003
Snoeijen-Schouwenaars FM, van Ool JS, Verhoeven JS, van Mierlo P, Braakman HMH, Smeets EE, Nicolai J, Schoots J, Teunissen MWA, Rouhl RPW, Tan IY, Yntema HG, Brunner HG, Pfundt R, Stegmann AP, Kamsteeg EJ, Schelhaas HJ, Willemsen MH (2019) Diagnostic exome sequencing in 100 consecutive patients with both epilepsy and intellectual disability. Epilepsia 60(1):155–164. https://doi.org/10.1111/epi.14618
Yuskaitis CJ, Ruzhnikov MRZ, Howell KB, Allen IE, Kapur K, Dlugos DJ, Scheffer IE, Poduri A, Sherr EH (2018) Infantile spasms of unknown cause: predictors of outcome and genotype-phenotype correlation. Pediatr Neurol 87:48–56. https://doi.org/10.1016/j.pediatrneurol.2018.04.012
Dhindsa RS, Bradrick SS, Yao X, Heinzen EL, Petrovski S, Krueger BJ, Johnson MR, Frankel WN, Petrou S, Boumil RM, Goldstein DB (2015) Epileptic encephalopathy-causing mutations in DNM1 impair synaptic vesicle endocytosis. Neurol Genet 1(1):e4. https://doi.org/10.1212/01.NXG.0000464295.65736.da
Epi KC (2016) De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am J Hum Genet 99(2):287–298. https://doi.org/10.1016/j.ajhg.2016.06.003
Lazzara A, Asghar S, Zacharia T, Byler D (2018) DNM1 mutation in a child associated with progressive bilateral mesial temporal sclerosis. Clin Case Rep 6(11):2037–2039. https://doi.org/10.1002/ccr3.1793
Naess K, Bruhn H, Stranneheim H, Freyer C, Wibom R, Mourier A, Engvall M, Nennesmo I, Lesko N, Wredenberg A, Wedell A, von Dobeln U (2021) Clinical presentation, genetic etiology, and coenzyme Q10 levels in 55 children with combined enzyme deficiencies of the mitochondrial respiratory chain J Pediatr 228:240–51 e2. https://doi.org/10.1016/j.jpeds.2020.08.025
Palmer EE, Schofield D, Shrestha R, Kandula T, Macintosh R, Lawson JA, Andrews I, Sampaio H, Johnson AM, Farrar MA, Cardamone M, Mowat D, Elakis G, Lo W, Zhu Y, Ying K, Morris P, Tao J, Dias KR, Buckley M, Dinger ME, Cowley MJ, Roscioli T, Kirk EP, Bye A, Sachdev RK (2018) Integrating exome sequencing into a diagnostic pathway for epileptic encephalopathy: evidence of clinical utility and cost effectiveness. Mol Genet Genomic Med 6(2):186–199. https://doi.org/10.1002/mgg3.355
Hamdan FF, Myers CT, Cossette P, Lemay P, Spiegelman D, Laporte AD, Nassif C, Diallo O, Monlong J, Cadieux-Dion M, Dobrzeniecka S, Meloche C, Retterer K, Cho MT, Rosenfeld JA, Bi W, Massicotte C, Miguet M, Brunga L, Regan BM, Mo K, Tam C, Schneider A, Hollingsworth G, Deciphering Developmental Disorders S, FitzPatrick DR, Donaldson A, Canham N, Blair E, Kerr B, Fry AE, Thomas RH, Shelagh J, Hurst JA, Brittain H, Blyth M, Lebel RR, Gerkes EH, Davis-Keppen L, Stein Q, Chung WK, Dorison SJ, Benke PJ, Fassi E, Corsten-Janssen N, Kamsteeg EJ, Mau-Them FT, Bruel AL, Verloes A, Ounap K, Wojcik MH, Albert DVF, Venkateswaran S, Ware T, Jones D, Liu YC, Mohammad SS, Bizargity P, Bacino CA, Leuzzi V, Martinelli S, Dallapiccola B, Tartaglia M, Blumkin L, Wierenga KJ, Purcarin G, O’Byrne JJ, Stockler S, Lehman A, Keren B, Nougues MC, Mignot C, Auvin S, Nava C, Hiatt SM, Bebin M, Shao Y, Scaglia F, Lalani SR, Frye RE, Jarjour IT, Jacques S, Boucher RM, Riou E, Srour M, Carmant L, Lortie A, Major P, Diadori P, Dubeau F, D’Anjou G, Bourque G, Berkovic SF, Sadleir LG, Campeau PM, Kibar Z, Lafreniere RG, Girard SL, Mercimek-Mahmutoglu S, Boelman C, Rouleau GA, Scheffer IE, Mefford HC, Andrade DM, Rossignol E, Minassian BA, Michaud JL (2017) High rate of recurrent de novo mutations in developmental and epileptic encephalopathies. Am J Hum Genet 101(5):664–685. https://doi.org/10.1016/j.ajhg.2017.09.008
Helbig KL, Farwell Hagman KD, Shinde DN, Mroske C, Powis Z, Li S, Tang S, Helbig I (2016) Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med 18(9):898–905. https://doi.org/10.1038/gim.2015.186
Joshi C, Kolbe DL, Mansilla MA, Mason SO, Smith RJ, Campbell CA (2016) Reducing the cost of the diagnostic odyssey in early onset epileptic encephalopathies. Biomed Res Int 2016:6421039. https://doi.org/10.1155/2016/6421039
Moller RS, Larsen LH, Johannesen KM, Talvik I, Talvik T, Vaher U, Miranda MJ, Farooq M, Nielsen JE, Svendsen LL, Kjelgaard DB, Linnet KM, Hao Q, Uldall P, Frangu M, Tommerup N, Baig SM, Abdullah U, Born AP, Gellert P, Nikanorova M, Olofsson K, Jepsen B, Marjanovic D, Al-Zehhawi LI, Penalva SJ, Krag-Olsen B, Brusgaard K, Hjalgrim H, Rubboli G, Pal DK, Dahl HA (2016) Gene panel testing in epileptic encephalopathies and familial epilepsies. Mol Syndromol 7(4):210–219. https://doi.org/10.1159/000448369
Rim JH, Kim SH, Hwang IS, Kwon SS, Kim J, Kim HW, Cho MJ, Ko A, Youn SE, Kim J, Lee YM, Chung HJ, Lee JS, Kim HD, Choi JR, Lee ST, Kang HC (2018) Efficient strategy for the molecular diagnosis of intractable early-onset epilepsy using targeted gene sequencing. BMC Med Genomics 11(1):6. https://doi.org/10.1186/s12920-018-0320-7
Kolnikova M, Skopkova M, Ilencikova D, Foltan T, Payerova J, Danis D, Klimes I, Stanik J, Gasperikova D (2018) DNM1 encephalopathy - atypical phenotype with hypomyelination due to a novel de novo variant in the DNM1 gene. Seizure 56:31–33. https://doi.org/10.1016/j.seizure.2018.01.020
Posey JE, Harel T, Liu P, Rosenfeld JA, James RA, Coban Akdemir ZH, Walkiewicz M, Bi W, Xiao R, Ding Y, Xia F, Beaudet AL, Muzny DM, Gibbs RA, Boerwinkle E, Eng CM, Sutton VR, Shaw CA, Plon SE, Yang Y, Lupski JR (2017) Resolution of disease phenotypes resulting from multilocus genomic variation. N Engl J Med 376(1):21–31. https://doi.org/10.1056/NEJMoa1516767
French CE, Dolling H, Megy K, Sanchis-Juan A, Kumar A, Delon I, Wakeling M, Mallin L, Agrawal S, Austin T, Walston F, Park SM, Parker A, Piyasena C, Bradbury K, Next Generation Children's Project C, Ellard S, Rowitch DH, Raymond FL (2022) Refinements and considerations for trio whole-genome sequence analysis when investigating Mendelian diseases presenting in early childhood HGG Adv 3 3 100113. https://doi.org/10.1016/j.xhgg.2022.100113
Parthasarathy S, Ruggiero SM, Gelot A, Soardi FC, Ribeiro BFR, Pires DEV, Ascher DB, Schmitt A, Rambaud C, Represa A, Xie HM, Lusk L, Wilmarth O, McDonnell PP, Juarez OA, Grace AN, Buratti J, Mignot C, Gras D, Nava C, Pierce SR, Keren B, Kennedy BC, Pena SDJ, Helbig I, Cuddapah VA (2022) A recurrent de novo splice site variant involving DNM1 exon 10a causes developmental and epileptic encephalopathy through a dominant-negative mechanism. Am J Hum Genet 109(12):2253–2269. https://doi.org/10.1016/j.ajhg.2022.11.002
Truty R, Patil N, Sankar R, Sullivan J, Millichap J, Carvill G, Entezam A, Esplin ED, Fuller A, Hogue M, Johnson B, Khouzam A, Kobayashi Y, Lewis R, Nykamp K, Riethmaier D, Westbrook J, Zeman M, Nussbaum RL, Aradhya S (2019) Possible precision medicine implications from genetic testing using combined detection of sequence and intragenic copy number variants in a large cohort with childhood epilepsy. Epilepsia Open 4(3):397–408. https://doi.org/10.1002/epi4.12348
Brereton E, Fassi E, Araujo GC, Dodd J, Telegrafi A, Pathak SJ, Shinawi M (2018) Mutations in the PH Domain of DNM1 are associated with a nonepileptic phenotype characterized by developmental delay and neurobehavioral abnormalities. Mol Genet Genomic Med 6(2):294–300. https://doi.org/10.1002/mgg3.362
Choi E, Dale B, RamachandranNair R, Ejaz R (2021) Pathogenic DNM1 gene variant presenting with unusually nonsevere neurodevelopmental phenotype a case report. Neurol Genet 7(5):e618. https://doi.org/10.1212/NXG.0000000000000618
van der Ven AT, Johannsen J, Kortum F, Wagner M, Tsiakas K, Bierhals T, Lessel D, Herget T, Kloth K, Lisfeld J, Scholz T, Obi N, Wortmann S, Prokisch H, Kubisch C, Denecke J, Santer R, Hempel M (2021) Prevalence and clinical prediction of mitochondrial disorders in a large neuropediatric cohort. Clin Genet 100(6):766–770. https://doi.org/10.1111/cge.14061
Devanna P, van de Vorst M, Pfundt R, Gilissen C, Vernes SC (2018) Genome-wide investigation of an ID cohort reveals de novo 3’UTR variants affecting gene expression. Hum Genet 137(9):717–721. https://doi.org/10.1007/s00439-018-1925-9
Sahly AN, Krochmalnek E, St-Onge J, Srour M, Myers KA (2020) Severe DNM1 encephalopathy with dysmyelination due to recurrent splice site pathogenic variant. Hum Genet 139(12):1575–1578. https://doi.org/10.1007/s00439-020-02224-5
Chappie JS, Acharya S, Leonard M, Schmid SL, Dyda F (2010) G domain dimerization controls dynamin’s assembly-stimulated GTPase activity. Nature 465(7297):435–440. https://doi.org/10.1038/nature09032
Damke H, Binns DD, Ueda H, Schmid SL, Baba T (2001) Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol Biol Cell 12(9):2578–2589. https://doi.org/10.1091/mbc.12.9.2578
Ford MG, Jenni S, Nunnari J (2011) The crystal structure of dynamin. Nature 477(7366):561–566. https://doi.org/10.1038/nature10441
Marks B, Stowell MH, Vallis Y, Mills IG, Gibson A, Hopkins CR, McMahon HT (2001) GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410(6825):231–235. https://doi.org/10.1038/35065645
Pucadyil TJ, Schmid SL (2008) Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135(7):1263–1275. https://doi.org/10.1016/j.cell.2008.11.020
Vallis Y, Wigge P, Marks B, Evans PR, McMahon HT (1999) Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis. Curr Biol 9(5):257–260. https://doi.org/10.1016/s0960-9822(99)80114-6
van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL (1993) Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol 122(3):553–563. https://doi.org/10.1083/jcb.122.3.553
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34(17):i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26(5):589–595. https://doi.org/10.1093/bioinformatics/btp698
Kim S, Scheffler K, Halpern AL, Bekritsky MA, Noh E, Kallberg M, Chen X, Kim Y, Beyter D, Krusche P, Saunders CT (2018) Strelka2: fast and accurate calling of germline and somatic variants. Nat Methods 15(8):591–594. https://doi.org/10.1038/s41592-018-0051-x
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20(9):1297–1303. https://doi.org/10.1101/gr.107524.110
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F (2016) The ensembl variant effect predictor. Genome Biol 17(1):122. https://doi.org/10.1186/s13059-016-0974-4
AlTassan R, AlQudairy H, Alromayan R, Alfalah A, AlHarbi OA, Gonzalez-Alvarez AC, Arold ST, Kaya N (2022) Clinical radiological and genetic characterization of a patient with a novel homoallelic loss-of-function variant in DNM1 Genes (Basel) 13 12 https://doi.org/10.3390/genes13122252
Yigit G, Sheffer R, Daana M, Li Y, Kaygusuz E, Mor-Shakad H, Altmuller J, Nurnberg P, Douiev L, Kaulfuss S, Burfeind P, Wollnik B, Brockmann K (2022) Loss-of-function variants in DNM1 cause a specific form of developmental and epileptic encephalopathy only in biallelic state. J Med Genet 59(6):549–553. https://doi.org/10.1136/jmedgenet-2021-107769
Ramachandran R, Surka M, Chappie JS, Fowler DM, Foss TR, Song BD, Schmid SL (2007) The dynamin middle domain is critical for tetramerization and higher-order self-assembly. EMBO J 26(2):559–566. https://doi.org/10.1038/sj.emboj.7601491
Asinof S, Mahaffey C, Beyer B, Frankel WN, Boumil R (2016) Dynamin 1 isoform roles in a mouse model of severe childhood epileptic encephalopathy. Neurobiol Dis 95:1–11. https://doi.org/10.1016/j.nbd.2016.06.014
Acknowledgements
We thank the patient’s family for participation in this study. We further thank Henrike Wilshusen and Elena Hamdorf for skilful technical assistance.
Funding
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (KU 1240/13–1 to K.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harms, F.L., Weiss, D., Lisfeld, J. et al. A deep intronic variant in DNM1 in a patient with developmental and epileptic encephalopathy creates a splice acceptor site and affects only transcript variants including exon 10a. Neurogenetics 24, 171–180 (2023). https://doi.org/10.1007/s10048-023-00716-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-023-00716-w