Abstract
In November 2019, Wuhan, a city in Central China, became the center of an outbreak of pneumonia of unknown cause, which was later named “coronavirus disease 2019” (COVID-19). COVID-19 is caused by the novel severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) infection. The emergence of novel SARS-CoV‑2 strains and mutations exerted a serious global public health threat. Although various vaccines have been developed, specific anti-SARS-CoV‑2 drugs are limited. As cardiologists, we believe that because SARS-CoV‑2 can bind to the angiotensin 2 receptor on the surface of cardiomyocytes, it may also lead to cardiac injury. COVID-19-associated cardiac injury is not rare in clinical practice, and most of these cases are mild, while a few might progress to fulminant myocarditis (FM). Overactivated immune response and inflammatory storm represent the core pathogenesis of COVID-19-associated FM. Early identification and diagnosis of COVID-19-associated FM are critical for its treatment. Recently, Wuhan was hit by the Omicron variant again. We proposed managing COVID-19-associated cardiac injury according to the severity, which has had a significant effect on outcome.
Zusammenfassung
Im November 2019 wurde Wuhan, eine Stadt in Zentralchina, zum Zentrum eines Ausbruchs von Pneumonien unbekannter Ursache, die später COVID-19 („coronavirus disease 2019“) genannt wurde. COVID-19 wird durch eine Infektion mit dem neuen, ein schweres akutes Atemnotsyndrom verursachenden Coronavirus 2 („severe acute respiratory distress syndrome coronavirus 2“, SARS-CoV-2) verursacht. Das Aufkommen neuartiger SARS-CoV-2-Stämme und -Mutationen stellte eine ernsthafte weltweite Bedrohung der öffentlichen Gesundheit dar. Es wurden zwar verschiedene Impfstoffe entwickelt, doch spezifische Anti-SARS-CoV-2-Medikamente sind nur begrenzt vorhanden. Als Kardiologen sind die Autoren der Ansicht, dass SARS-CoV‑2 – weil es an den Angiotensin-2-Rezeptor auf der Oberfläche von Kardiomyozyten binden kann – möglicherweise auch zu kardialen Läsionen führt. COVID-19-assoziierte kardiale Läsionen sind im klinischen Alltag nicht selten, und die meisten dieser Fälle sind von mittlerer Schwere, jedoch könnten einige wenige zu einer fulminanten Myokarditis (FM) fortschreiten. Eine überaktivierte Immunantwort und ein inflammatorischer Sturm stellen die Kernbestandteile der Pathogenese einer COVID-19-assoziierten FM dar. Die frühzeitige Erkennung und Diagnose einer COVID-19-assoziierten FM sind für ihre Behandlung entscheidend. Vor Kurzem gab es in Wuhan erneut einen Ausbruch der Omikron-Variante. Die Autoren schlugen vor, die COVID-19-assoziierten kardialen Läsionen nach ihrer Schwere zu behandeln, was einen signifikanten Effekt auf das Ergebnis hatte
Similar content being viewed by others
On January 8, 2023, after China’s 3 years of strict measurements for the prevention and control of the epidemic caused by severe acute respiratory distress syndrome corona virus 2 (SARS-CoV-2)-induced coronavirus disease 2019 (COVID-19), COVID-19 was downgraded to “Class B infectious diseases.” After the lifting of epidemic control, the Omicron strain spread rapidly among the population. Recently, our department also received patients with cardiac injury or even fulminant myocarditis (FM) after infection with the Omicron strain. There are also many cases of sudden cardiac death after COVID-19 in the news media, which has led to widespread concern and discussion among the population. This review briefly introduces the recognition, diagnosis, and treatment of recent COVID-19-associated myocarditis in China, especially with our experience in Wuhan.
SARS-CoV-2 general characteristics and cardiac injury
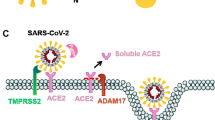
SARS-CoV‑2 belongs to betacoronavirus with a capsule, and its particles are round or oval, with a diameter of 60–140 nm. The virus particles contain four structural proteins: the spike protein, envelope protein, membrane protein, and nucleocapsid protein. The genome of SARS-CoV‑2 is a positive-sense single-stranded RNA with a total length of 29.9 kb. The nucleocapsid protein wraps the viral RNA to form the core structure of the viral particles, named the “nucleocapsid.” The nucleocapsid is wrapped by a double lipid membrane inlaid with the SARS-CoV‑2 spike protein, membrane protein, and nucleocapsid protein [1]. SARS-CoV‑2 gene mutations occur frequently during human transmission. To date, the World Health Organization has proposed five “variants of concern (VOC),” including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529). The Omicron variant appeared in the population in November 2021. Compared with Delta and other VOC, its transmissibility and immune escape ability were significantly enhanced. It quickly took over the Delta variant in early 2022 and became the dominant epidemic strain in the world. In early 2023, five subtypes of Omicron (BA.1, BA.2, BA.3, BA.4, BA.5) were prevalent in China, and have evolved into 709 sub-branches, including 72 recombination branches.
Multiple evidence showed that the lung pathogenicity of the Omicron variant was significantly weakened, and its clinical manifestation had changed from pneumonia to upper respiratory tract infection [2]. Because angiotensin-converting enzyme 2 (ACE2) receptors are widely distributed in cardiomyocytes, gastrointestinal epithelial cells, vascular endothelial cells, and other cell types, SARS-CoV‑2 can also invade the heart to cause myocarditis or the gastrointestinal tract to cause gastroenteritis. At the beginning of 2020, in the cardiac autopsy analysis of 26 cases with critical illness caused by infection with the original SARS-CoV‑2 strain, Zhang et al. found that neutrophil infiltration in myocardial tissue was closely associated with the severity of myocardial damage and the progress of COVID-19 infection, [3]. In addition to direct myocardial infection, inflammatory (or cytokine) storms caused by infected lung and other tissues can also result in myocarditis and cardiac injury.
Incidence of COVID-19-associated myocarditis
Myocarditis refers to myocarditis injury resulting from various reasons. Viral infection is the most common etiology, such as coxsackie virus, adenovirus, and influenza virus. In recent years, in the COVID-19 pandemic, myocarditis induced by SARS-CoV‑2 infection has been very common [4,5,6,7]. According to the patient’s hemodynamic stability, COVID-19-associated myocarditis can be divided into acute myocarditis and fulminant myocarditis (FM). Similar to the FM caused by other pathogenic infections, COVID-19-associated FM has a sudden onset and rapid progression. The patients soon present with hemodynamic abnormalities and serious arrhythmia, which might be secondary to or accompanied by respiratory, liver, and kidney failure. The early mortality rate is very high. According to the New England Journal of Medicine, before the COVID-19 epidemic, the global incidence rate of myocarditis was about 1–10 cases/100,000 people per year. In the 35–39-year age group, the incidence rate is 6.1 cases per 100,000 (male) and 4.4 cases per 100,000 (female). The incidence rate in the 20–44-year age group is similar. During the COVID-19 pandemic, 2.4 of every 1000 patients hospitalized for COVID-19 were diagnosed with, or highly suspected of having, myocarditis [8, 9]. Among the recently admitted patients with Omicron infection to our department, the internal medicine department of Tongji Hospital affiliated with the Tongji Medical College of Huazhong University of Science and Technology, cardiac troponin I increased by 8–10%. Male patients, adults older than 50 years, and children younger than 16 years are high risk factors for COVID-19-associated myocarditis (Fig. 1).
Profile of patients with COVID-19-associated myocarditis during the Omicron wave in Tongji Hospital. a The proportion of patients with COVID-19-associated myocarditis during the Omicron wave in Tongji Hospital (a total of 3158 patients with COVID-19). b The proportion of acute myocarditis and fulminant myocarditis in patients with COVID-19 (a total of 128 COVID-19 patients with myocarditis). Diagnosis of COVID-19-associated acute myocarditis and fulminant myocarditis was based on the following criteria: (1) for laboratory confirmation of COVID-19, nasal and pharyngeal swabs must show at least two consecutive positive results from high-throughput sequencing or real-time reverse transcriptase–polymerase chain reaction assays; (2) histologically proven borderline (presence of inflammatory infiltrate) or active (presence of inflammatory infiltrate plus myocardial necrosis) myocarditis according to the Dallas criteria; (3) acute presentation, defined by the onset of cardiac symptoms up to 30 days before admission; (4) hs-cTnI > 26.2 pg/mL at admission. Patients with any prior diagnosis of myocardial disease were excluded. (5) In addition to (1)–(4), fulminant myocarditis was diagnosed on the basis of documented left ventricular dysfunction (LVEF < 50%) and low cardiac output syndrome requiring inotropes or mechanical circulatory support (intra-aortic balloon pump and/or extracorporeal membrane oxygenation) according to Chinese Society of Cardiology expert consensus statement on the diagnosis and treatment of adult fulminant myocarditis [26, p. 187]; the remaining cases were classified as acute myocarditis
Overactivated immune response and inflammatory storm: core pathogenesis of COVID-19-associated FM
SARS-CoV-2 invasion of the host cell
After SARS-CoV‑2 invades the respiratory tract, spike protein is the key for the virus to bind to the ACE2 receptor on the surface of the host cell. Its main function is to recognize the receptor on the surface of the host cell and mediate the fusion of the virus and the host cell [10]. ACE2 receptors are widely distributed in alveolar epithelium, intestinal epithelium, myocardial cells, renal tubular epithelium, and vascular endothelial cells [11], which might lead to the related clinical symptoms of the host. Therefore, the host might present with symptoms related to the lung, gastrointestinal tract, and cardiovascular system after being infected with SARS-CoV‑2 [12]. Autopsy studies showed that a large number of lymphocytes infiltrated the lung, heart, kidney, and liver of patients who died of COVID-19. SARS-CoV‑2 was detected in tissues from the myocardium and alveolar epithelium by electron microscopy [13], suggesting that COVID-19 could directly invade the myocardium and alveolar tissue.
Immune response
In the early stage of infection, if the host’s immune response is rapid and strong enough, when the virus enters the upper respiratory tract or the lungs via aerosols, the local innate immune system can clear the virus before it spreads. Thus, the infected person may not have clinical symptoms or have only mild symptoms. If the virus is not cleared, it will enter a rapid replication period in the body. In the case of a weak immune response, the patient may become an asymptomatic carrier and subsequently spread the virus. Some patients have a very strong immune response to the virus, which may induce an “inflammatory storm” with obvious symptoms [14].
Inflammatory storm
Once the inflammatory storm has started, it will continue to cascade and amplify, eventually causing multiorgan inflammatory damage and systemic symptoms. Cytokines, mainly interleukin (IL), tumor necrosis factor, interferon, and colony-stimulating factor, are secreted by immune cells (such as monocytes, macrophages, T cells, B cells, natural killer cells, etc.) and some non-immune cells (endothelial cells, epidermal cells, fibroblasts, etc.). Through chemotaxis and adhesion, cytokines recruit inflammatory cells into the inflammatory location, which in turn release inflammatory mediators, activate more inflammatory cells, and create an inflammatory storm until the pathogen is eliminated or the patient dies [15]. Some inflammatory mediators damage vascular endothelial cells. The damage or contraction of vascular endothelial cells stimulated by inflammatory mediators increases the permeability of the capillary wall, resulting in the leakage of capillaries, thus entering the most severe phase in the later stage of the inflammatory storm, namely, leakage syndrome, which is very difficult to treat clinically and is associated with a high mortality rate [16, 17]. Inflammation-related substances that promote the increase of vascular permeability mainly include pro-inflammatory cytokines, chemotactic cytokines, leukotrienes, prostaglandins, platelet-activating factors, complement, histamine, bradykinin, reactive oxygen species, and nitric oxide [18]. Compared with non-critical patients with COVID-19, the plasma levels of IL‑2, IL‑7, IL-10, and granulocyte colony-stimulating factor were significantly higher in critical patients with COVID-19, suggesting that there is a positive correlation between the level of cytokines and the severity of COVID-19 [19]. In addition, CD4+ T cells of patients with COVID-19 will be rapidly activated, producing a large number of granulocyte macrophage colony-stimulating factor and IL‑6. Granulocyte macrophage colony-stimulating factor will further activate CD14+ and CD16+ inflammatory monocytes to produce more IL‑6, which in turn enhance the damage of target organs [20, 21].
In the 22 autopsies of 277 patients with COVID-19, typical myocarditis accounted for 7.2%, non-myocarditis inflammatory infiltration accounted for 12.6%, necrosis, injury, or local necrosis of single myocardial cells accounted for 13.7%, and acute myocardial infarction accounted for 4.7% of cases, while 47.8% of patients had at least one cardiovascular histopathological abnormality, including large vessel or microvascular thrombosis, inflammation, or intracavitary megakaryocytes. These data indicate that nonspecific cardiac inflammation and/or injury are common in fatal cases of COVID-19 [8].
Early diagnosis and treatment of COVID-19-associated myocarditis
The diagnostic criteria of COVID-19-associated myocarditis are similar to those of myocarditis resulting from other causes [22,23,24]. Because of the rapid spread of Omicron, we have recently witnessed a sudden increase of patients with COVID-19-associated myocarditis. COVID-19-associated myocarditis is suspected when a patient with SARS-CoV‑2 nucleic acid or antigen positive results has the following conditions: (1) a sudden onset of fever, fatigue, cough, loss of appetite, or diarrhea and other symptoms; (2) laboratory examination shows increased levels of hypersensitive cardiac troponin I/cardiac troponin I or B‑type natriuretic peptide/N-terminal B‑type natriuretic peptide; (3) obvious changes on the ECG (low voltage, wide lead ST segment, T wave changes, and conduction block, etc.). If serious hemodynamic disorder (including hypotension or shock) or arrhythmia (malignant arrhythmia such as atrioventricular block, sinus tachycardia, ventricular tachycardia, or ventricular fibrillation) occur rapidly, COVID-19-associated FM should be considered. If the echocardiogram shows diffuse decrease in ventricular wall motion, significant decrease in left ventricular ejection fraction (LVEF), and decrease in left ventricular long-axis strain, and if the level of inflammatory factors is significantly increased, the patient can be clinically diagnosed as having COVID-19-associated myocarditis after excluding acute myocardial infarction, stress cardiomyopathy, and other acute myocardial injuries.
In some patients with COVID-19-associated myocarditis, hypersensitive cardiac troponin I/cardiac troponin I and B‑type natriuretic peptide/N-terminal B‑type natriuretic peptide did not increase significantly in the early stage. After excluding electrolyte disorders and hereditary ion channel diseases, these patients should undergo inflammatory factor detection, echocardiography, myocardial biopsy, cardiac magnetic resonance imaging, or fluorine-18-deoxyglucose positron emission tomography as soon as possible to make a definite diagnosis. It is worth mentioning that echocardiography, as a simple and accessible noninvasive examination in outpatient and inpatient wards, is very characteristic in the early diagnosis of patients with myocarditis. The common sign is the significant decrease of overall ventricular wall motion, while segmental motion differences might also be observed. The LVEF of patients with FM decreased rapidly in the early stage. Our department carried out two-dimensional spot tracking echocardiographic left ventricular strain analysis on severe and critical patients with COVID-19. We found that patients with COVID-19 have extensive left ventricular systolic function impairment in the early stage, which was positively related to the severity of the disease [25]. In the follow-up it was revealed that after careful treatment, the left ventricular strain analysis and ejection fraction of these patients with myocarditis recovered well (Fig. 2).
Cardiac function of patients with COVID-19-associated myocarditis during the Omicron wave in Tongji Hospital. a Comparison of left ventricular ejection fraction (LVEF) on admission between patients with COVID-19-associated acute myocarditis (n = 60) and patients with COVID-19-associated fulminant myocarditis (n = 11). b Comparison of LVEF between admission and discharge in patients with COVID-19-associated fulminant myocarditis (n = 11). Black dots indicate the LVEF of patients with COVID-19-associated acute myocarditis on admission, red dots indicate the LVEF of patients with COVID-19-associated fulminant myocarditis on admission, blue dots indicate the LVEF of patients with COVID-19-associated fulminant myocarditis on discharge. ∗∗p < 0.01, ∗∗∗∗p < 0.0001
Active treatment of COVID-19-associated myocarditis
We found that most cases of COVID-19-associated myocarditis were acute and common, while a few were severe FM. Due to the lack of specific drugs for COVID-19, the “life support-based comprehensive treatment regimen” remains important in the treatment of COVID-19-associated FM to achieve “early identification, early diagnosis, early prediction, and early treatment” [26, 27]. In Wuhan, the myocardial injury markers were detected for every inpatient, the diagnosis of cardiac injury or FM was made based on the medical history, the risk stratification was made according to the severity of the patient’s condition, and the classification management was carried out for patients with different risk levels.
Treatment of acute common COVID-19-associated myocarditis
Treatment comprises mainly general symptomatic and supportive treatment, including:
-
1.
Absolute bed rest, reduce visits, and avoid emotional stimulation and fluctuation; offer a light, digestible, and nutritious diet, multiple small meals, or supplement nutrition through nasal feeding tube.
-
2.
Nasal catheter, mask oxygen inhalation, or positive pressure oxygen supply to improve hypoxemia or respiratory failure.
-
3.
Immunomodulatory therapy represented by glucocorticoids and gamma globulin.
-
4.
COVID-19 patients with SARS-CoV‑2 nucleic acid positivity can be additionally treated with antiviral therapy such as nirmatrelvir/ritonavir tablets or azvudine.
-
5.
Trimetazidine and other drugs that can improve myocardial energy metabolism could be added, beta-blockers and angiotensin-converting enzyme inhibitors could also be added later.
-
6.
If the diet is poor, it is necessary to supplement water-soluble and fat-soluble vitamins to help prevent disseminated intravascular coagulation.
-
7.
Use proton pump inhibitor to prevent stress ulcer and gastrointestinal bleeding.
Treatment of acute common COVID-19-associated FM
The prominent problems of patients with FM are acute circulatory failure and cardiogenic shock, which should be treated according to the “life support-based comprehensive treatment regimen” [26, 27], with the following key points.
Mechanical life support
Use mechanical circulatory devices to maintain circulation stability, ensure organ perfusion, and rest the failed heart, rather than cardiotonic and vasoactive drugs to raise blood pressure. If necessary, ventilator-assisted breathing, temporary pacemaker implantation, and blood purification treatment should be offered.
Mechanical circulation support measurements include:
-
1.
Intra-aortic balloon pump (IABP).
-
2.
Impella, left ventricular assist device, or artificial heart, etc.
-
3.
Extracorporeal membrane oxygenation (ECMO). The application of veno-arterial ECMO in the treatment of FM has been supported by a large number of clinical data. If the patient’s condition is complicated with respiratory failure, venous-venous ECMO or venous-arterial-venous ECMO can be considered. It is recommended to use ECMO immediately if IABP or Impella is still insufficient to maintain the circulatory disorders. If the patient has serious circulatory disturbance, serious left ventricular insufficiency, or cardiac arrest requiring cardiopulmonary resuscitation, ECMO should be started immediately, and an IABP might be placed at the same time.
Other mechanical life support treatments include mechanical ventilation, continuous renal replacement therapy, and temporary pacing therapy.
Common mechanical ventilation measurements include:
-
1.
Noninvasive mechanical ventilation, which is recommended for COVID-19-associated FM patients with hypoxemia/respiratory failure, a breathing rate > 20 times/min, who can fully cooperate with and adapt to ventilator ventilation, and are expected to have short-term remission. Noninvasive mechanical ventilation is prohibited for patients with unclear consciousness and impaired airway self-cleaning ability. When applying noninvasive ventilation, the vital signs and treatment reactions of patients should be closely monitored, and invasive mechanical ventilation should be used if necessary.
-
2.
Invasive mechanical ventilation: it should be applied when hypoxemia patients cannot adapt to noninvasive mechanical ventilation or cardiopulmonary resuscitation, or when noninvasive ventilation support is insufficient. For FM, the frequency of invasive mechanical ventilation is significantly increased compared with other diseases.
-
3.
High-flow transnasal oxygen therapy: for patients with hypoxemia/acute type I respiratory failure, it is recommended to use high-flow nasal oxygen therapy instead of traditional oxygen therapy to reduce the tracheal intubation rate and the need for respiratory support upgrading.
The main purpose of continuous renal replacement therapy is to remove toxins and inflammatory factors from patients with FM continuously in order to reduce the damage caused by the inflammatory storm, and to help maintain the body fluid and acid–base balance and stabilize the internal environment. It should be used as early as possible when combined with renal function injury or acute left heart failure. Immunoadsorption may help remove inflammatory factors more effectively.
Immunomodulation therapy
Maintaining immune balance is the fundamental strategy. In FM, excessive immune activation and inflammatory storm lead to serious cardiac damage. Based on this core pathophysiological process, immunomodulation treatment, including adequate doses of gamma globulin, instead of immunosuppression treatment using cytotoxic drugs is recommended. Monoclonal antibodies to cytokines can be used if necessary, which can block the key target of pathogenesis, reduce inflammatory edema, is anti-shock, relieve clinical symptoms, save patients’ lives, and improve the prognosis.
It is recommended to start intravenous injection of gamma globulin 10–20 g/day as early as possible after admission, and reduce it by half to 5–10 g after 3–5 days of use, and continue to use it for 3–5 days, with a total amount of approximately 2 g/kg [28].
Tocilizumab is a recombinant humanized IL‑6 receptor monoclonal antibody of immunoglobulin G1 subtype, which plays a role by imitating the natural antibody produced by the immune system. It can be used for critical patients with significantly elevated levels of IL‑6. The first dose is 4–8 mg/kg, the recommended dose is 400 mg diluted to 100 mL, and the infusion time is more than 1 h. For cases where there is poor efficacy in the first administration, an additional application (the same dose as before) can be made 12 h after the first administration. The cumulative number of administrations is up to 2 times, and the maximum amount of a single dose is not more than 800 mg. Attention should be paid to allergic reactions. It is forbidden for patients with active infection such as tuberculosis.
Antiviral treatment
Antiviral drugs can be used for SARS-CoV‑2 to block virus replication, reduce viral load, and reduce direct and indirect immune damage to the heart caused by the virus.
-
1.
The combination of nirmatrelvir/ritonavir is applicable to adult patients with mild and moderate COVID-19 and high-risk factors for critical progression within 5 days.
-
2.
Azvudine may be used to treat adult patients with moderate COVID-19.
-
3.
Molnupiravir is recommended for adult patients with mild-to-moderate COVID-19 and high-risk factors for progression to severe COVID-19 within 5 days of onset.
-
4.
Monoclonal antibody (amubarvimab/romlusevimab injection) could be used in combination to treat adults and adolescents (12–17 years old, weight ≥ 40 kg) with mild-to-moderate COVID-19 and high-risk factors for progression to severe COVID-19.
Gradual rehabilitation of COVID-19-associated myocarditis
The relative risk rate of COVID-19-associated myocarditis was highest in the first week of COVID-19 onset, and then gradually decreased. Therefore, it is necessary to have good rest within 1–2 weeks of COVID-19 onset, and not to take part in strenuous exercise for 1 month. After 2 weeks of rehabilitation, patients can first try low-intensity exercise, such as walking, housework, respiratory training to restore lung function, stretching exercise to improve flexibility, balance and yoga exercise to improve stability, and control the heart rate below 70% of the maximum heart rate (220—age) for no more than 15 min, and then gradually increase the amount of exercise until it is back to the pre-COVID-19 level. Patients with COVID-19-associated myocarditis should stop strenuous exercise for 3–6 months. After 3–6 months, it is necessary to further assess the recovery plan after the evaluation of myocardial enzymes, ECG, 24‑h dynamic ECG, and/or cardiac magnetic resonance imaging [29].
Conclusion
During the 3 years of the COVID-19 pandemic, the identification, diagnosis, and active treatment of COVID-19-associated myocarditis have been particularly urgent matters. An overactivated immune response and inflammatory storm are the core pathogenesis of COVID-19-associated fulminant myocarditis (FM). On the basis of the “life support-based comprehensive treatment regimen,” we managed to save the lives of many patients with COVID-19-associated FM.
References
Du H, Wang DW, Chen C (2020) The potential effects of DPP‑4 inhibitors on cardiovascular system in COVID-19 patients. J Cell Mol Med 24:10274–10278
Wang LY, Zhao CX, Wang F, Miao K, Wang DW (2023) Early identification, diagnosis and treatment of COVID-19 related myocarditis, save more lives. Zhonghua Xin Xue Guan Bing Za Zhi 51:1–5
Zhang Q, Zhang H, Yan X et al (2022) Neutrophil infiltration and myocarditis in patients with severe COVID-19: A post-mortem study. Front Cardiovasc Med 9:1026866
Chen C, Zhou Y, Wang DW (2020) SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 45:230–232
Albert CL, Carmona-Rubio AE, Weiss AJ, Procop GG, Starling RC, Rodriguez ER (2020) The enemy within: sudden-onset reversible cardiogenic shock with biopsy-proven cardiac myocyte infection by severe acute respiratory syndrome Coronavirus 2. Circulation 142:1865–1870
Cui G, Li R, Zhao C, Wang DW (2021) Case report: COVID-19 vaccination associated fulminant myocarditis. Front Cardiovasc Med 8:769616
Liu PP, Kafil TS (2022) COVID-19 vaccine myocarditis: cautious reassurance in an era of dynamic uncertainty. J Am Coll Cardiol 80:2266–2268
Ammirati E, Lupi L, Palazzini M et al (2022) Prevalence, characteristics, and outcomes of COVID-19-associated acute myocarditis. Circulation 145:1123–1139
Basso C (2022) Myocarditis. N Engl J Med 387:1488–1500
Shang J, Ye G, Shi K et al (2020) Structural basis of receptor recognition by SARS-CoV‑2. Nature 581:221–224
Wang JJ, Edin ML, Zeldin DC, Li C, Wang DW, Chen C (2020) Good or bad: application of RAAS inhibitors in COVID-19 patients with cardiovascular comorbidities. Pharmacol Ther 215:107628
Chen C, Wang F, Chen P et al (2020) Mortality and pre-hospitalization use of renin-angiotensin system inhibitors in hypertensive COVID-19 patients. J Am Heart Assoc 9:e17736
Tian S, Xiong Y, Liu H et al (2020) Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol 33:1007–1014
Chen C, Li H, Hang W, Wang DW (2020) Cardiac injuries in coronavirus disease 2019 (COVID-19). J Mol Cell Cardiol 145:25–29
Li H, Chen C, Wang DW (2021) Inflammatory cytokines, immune cells, and organ interactions in heart failure. Front Physiol 12:695047
Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y (2021) The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Target Ther 6:255
Hang W, Chen C, Seubert JM, Wang DW (2020) Fulminant myocarditis: a comprehensive review from etiology to treatments and outcomes. Signal Transduct Target Ther 5:287
Cheung PC, Eisch AR, Maleque N, Polly DM, Auld SC, Druey KM (2021) Fatal exacerbations of systemic capillary leak syndrome complicating Coronavirus disease. Emerg Infect Dis 27:2529–2534
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Zhou Y, Fu B, Zheng X et al (2020) Pathogenic T‑cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 7:998–1002
Ascierto PA, Fu B, Wei H (2021) IL‑6 modulation for COVID-19: the right patients at the right time? J Immunother Cancer 9(4):e002285. https://doi.org/10.1136/jitc-2020-002285
Zhuang Y, Wang J, Li H, Chen Y, Chen C, Wang DW (2022) Plasma Siglec‑5 and CD163 as novel biomarkers for fulminant myocarditis. Biomedicines 10(11):2941. https://doi.org/10.3390/biomedicines10112941
Wang J, He M, Li H et al (2022) Soluble ST2 is a sensitive and specific biomarker for fulminant myocarditis. J Am Heart Assoc 11:e24417
Nie X, He M, Wang J et al (2020) Circulating miR-4763-3p is a novel potential biomarker candidate for human adult fulminant myocarditis. Mol Ther Methods Clin Dev 17:1079–1087
Li R, Wang H, Ma F et al (2021) Widespread myocardial dysfunction in COVID-19 patients detected by myocardial strain imaging using 2‑D speckle-tracking echocardiography. Acta Pharmacol Sin 42:1567–1574
Wang D, Li S, Jiang J et al (2019) Chinese society of cardiology expert consensus statement on the diagnosis and treatment of adult fulminant myocarditis. Sci China Life Sci 62:187–202
Li S, Xu S, Li C et al (2019) A life support-based comprehensive treatment regimen dramatically lowers the in-hospital mortality of patients with fulminant myocarditis: a multiple center study. Sci China Life Sci 62:369–380
Bemtgen X, Klingel K, Hufnagel M et al (2021) Case report: lymphohistiocytic myocarditis with severe cardiogenic shock requiring mechanical cardiocirculatory support in multisystem inflammatory syndrome following SARS-coV‑2 infection. Front Cardiovasc Med 8:716198
Salman D, Vishnubala D, Le Feuvre P et al (2021) Returning to physical activity after covid-19. BMJ 372:m4721
Acknowledgements
We thank our colleagues from the Division of Cardiology, Tongji Hospital for their tremendous efforts during this investigation.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82241034 and 82270363). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Chen, W. He and D.W. Wang declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Rights and permissions
About this article
Cite this article
Chen, C., He, W. & Wang, D.W. Wuhan 3 years after the outbreak of the pandemic—cardiological insights and perspectives. Herz 48, 173–179 (2023). https://doi.org/10.1007/s00059-023-05176-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-023-05176-4