Abstract
In Candida parapsilosis, homozygous disruption of the two genes encoding trehalase activity increased the susceptibility to Itraconazole compared with the isogenic parental strain. The fungicidal effect of this azole can largely be counteracted by preincubating growing cells with rotenone and the protonophore 2,4-Dinitrophenol. In turn, measurement of endogenous reactive oxygen species formation by flow cytometry confirmed that Itraconazole clearly induced an internal oxidative stress, which can be significantly abolished in rotenone-exposed cells. Analysis of the antioxidant enzymatic activities of catalase and superoxide dismutase pointed to a moderate decrease of catalase in trehalase-deficient mutant cells compared to the wild type, with an additional increase upon addition of rotenone. These enzymatic changes were imperceptible in the case of superoxide dismutase. Alternative assays with Voriconazole led to a similar profile in the results regarding cell growth and antioxidant activities. Collectively, our data suggest that the antifungal action of Itraconazole on C. parapsilosis is dependent on a functional mitochondrial activity. They also suggest that the central metabolic pathways in pathogenic fungi should be considered as preferential antifungal targets in new research.
Similar content being viewed by others
Introduction
The amazing spread of mycotic infections that affect the immnucompromised population (i.e., aging people, neonates, AIDS patients and those subjected to invasive surgery, prolonged treatments with antibiotics or suffering chronic diseases, etc.) and the rising identification in both community and hospitality epidemic outbreaks of fungal species traditionally considered as innocuous (Pfaller and Diekema 2010; Pfaller et al. 2014; Brown et al. 2012; Kainz et al. 2020) conveys additional complications for clinical antifungal chemotherapy, already limited by the scarce availability of true fungicidal compounds and the growing isolation of strains which display resistance to the conventional antimycotics (Kontoyiannis Lewis 2002, Denning and Bromeley 2015, Campoy and Adrio 2017).
The taxonomical complex Candida parapsilosis may be considered a significant example of this worrying scenario. The clinical incidence of this emergent yeast has increased in the last years and represents the second or third most common opportunistic fungal pathogen isolated worldwide (Trofa et al. 2008; Toth et al. 2019). As regards the antifungal treatments for C. parapsilosis, some specific pathobiological features must be borne in mind. For example, this yeast does not form true hyphae and is able to colonize the skin and produce stable biofilms on plastic-made devices, with a high incidence in neonates (Holland et al. 2014; Pal et al. 2021). As a general rule, although susceptibility testing is recommended for all clinical isolates, azoles appear to be useful, despite some reports of resistance whereas echinocandins have higher MICs values and display lower in vitro toxic activity compared to other Candida species (Cantón et al. 2011; Zhang et al. 2015). In turn, the new formulations of amphotericin B overcome the hepatic and renal toxicity caused by the classical formulations of this polyene and can be applied in adults (Saliba and Dupont 2008).
As a complementary strategy, the central nutritional pathways in pathogenic fungi have recently been proposed as preferential therapeutic candidates for new investigations into antifungals (Prieto et al. 2014; Wijnants et al. 2020; 2022). Within this area, the enzymatic routes involved in trehalose metabolism have received great attention since this non-reducing disaccharide is present in bacteria, fungi, plants and invertebrates, but is surprisingly absent from vertebrates, which, in turn, contain two trehalase isoforms responsible for the hydrolysis of trehalose ingested in the diet (Tournu et al. 2013; Perfect et al. 2017, Argüelles 2017; Thamagong et al. 2017). Furthermore, the genes encoding trehalose biosynthesizing enzymes (trehalose synthase and trehalose phosphatase) are factors of virulence and the corresponding null mutants display a phenotype of highly susceptibility to oxidative and saline stress exposure, also reported in C. albicans and budding yeasts (Pedreño et al. 2002; Van Dijck et al. 2002; Garre and Matallana 2009).
In our previous studies on trehalose metabolism in C. parapsilosis, we disrupted the two single genes encoding trehalase activity (ATC1/NTC1) in this yeast, and demonstrated their specific role in virulence (Sánchez-Fresneda et al. 2015). Thus, the homozygous atc1Δ/ntc1Δ null mutant, besides its inability to mobilize trehalose, displays a pleiotropic phenotype that includes the impairment of cell growth, resistance to nutrient starvation and oxidative stress, together with a reduction of virulence and the capacity to form biofilms (Sánchez-Fresneda et al. 2015). A recent analysis of azole susceptibility showed that atc1Δ/ntc1Δ cells were more sensitive to itraconazole (ITC) than the parental strain (Sánchez-Fresneda et al. 2022). We report here that the ITC-induced fungicidal effect is dependent on a functional mitochondrial activity.
Materials and methods
Strains and growth conditions
The C. parapsilosis strains used in this study have been reported elsewhere (Sánchez-Fresneda et al. 2015). Liquid cultures were grown at 37°C by shaking in YPD medium consisting of 2% peptone, 1% yeast extract and 2% glucose. Solid media contained 2% agar. The time-course growth in liquid medium was measured by monitoring cell density at 600 nm (OD600) or by direct cell counting in a TC-20 cell counter (BioRad). Cell viability was determined in samples diluted appropriately with sterile water b plating in triplicate on solid YPD after incubation for 1–2 days at 37°C. Between 30 and 300 colonies were counted per plate. Survival rates were normalized to control samples (100% viability). Colony growth in solid medium was tested by spotting 5 μl from the respective tenfold dilutions onto YPD agar. The plates were then incubated at 30°C and scored after 24 or 48 h.
Preparation of cell-free extracts
After exposure to the indicated treatments, samples from YPD liquid cultures were harvested, washed and resuspended at known cell densities (10–15 mg/ml, wet weight) in the extraction buffer, 100 mM 4-morpholine-ethanesulfonic acid (MES) pH 6.0, containing 5 mM cysteine and 0.1 mM phenyl methyl sulphonyl fluoride (PMSF). Cellular suspensions were transferred into small pre-cooled tubes (1.0 cm-diameter) with 1.5 g Ballotini glass beads (0.45 mm diameter). The cells were broken by vigorously vibrating the tubes in a vortex mixer and rapidly cooled in ice. The crude extract was then centrifuged at 10,000 ×g for 5 min and the pellet was resuspended in the same buffer at the initial density. For antioxidant assays, the supernatant fraction obtained was filtered through Sephadex G-25 NAP columns (Amersham Pharmacia Biotech AB) previously equilibrated with 50 mM K-phosphate buffer, pH 7.8, to remove low-molecular-weight compounds that could interfere with the measurements.
Enzymatic assays
Catalase activity was determined at 240 nm by monitoring the removal of H2O2 following the protocol described elsewhere (González-Parraga et al. 2011). Measurements of superoxide dismutase (SOD) were carried out spectrophotochemically by the ferricytochrome C method using xanthine/xanthine oxidase as the source of superoxide anions (O2.−) (González-Parraga et al. 2011). Protein was estimated using the Lowry method (1953) with bovine serum albumin as standard. Data of enzymatic activity were normalized in relation to a control measurement (100%).
ROS determination
Intracellular ROS formation by flow cytometry with dihydrofluorescein diacetate (DHF), was measured following the procedure described in Sangalli-Leite et al. (2011) with the additional modifications indicated elsewhere (Sánchez-Fresneda et al. 2015). Fluorescence intensity was determined using the EPICS XLMCL4 cytometer (Beckman Coulter) equipped with an argon ion laser with an excitation power of 15 mW at 488 nm. Forward scatter (FSC) and side scatter (SSC) were analyzed on linear scales, while the analyses of green (FL1) fluorescence intensity were made on logarithmic scale. Analysis gates were set around debris and intact cells, on an FSC vs SSC dot plot. Fluorescence histograms corresponding to 5000 cells were generated using the gated data. Data acquisition and analysis were performed using WINMDI software (available from http://facs.scripps.edu).
Results
The antifungal effect of itraconazole on C. parapsilosis is counteracted by the presence of rotenone and DNP
Neutral and acid trehalase activities in Candida parapsilosis are coded by two distinct genes, called NTC1 and ATC1 respectively, which are non-essential genes (Sánchez-Fresneda et al. 2015). Therefore, a double atc1Δ/ntc1Δ null mutant obtained by homozygous deletion of both genes is perfectly viable, although atc1Δ/ntc1Δ glucose-grown cultures displayed a weak phenotype of cell death after prolonged incubation in stationary phase (5 days onwards) (Sánchez-Fresneda et al. 2015). However, for the purpose of the ITC-induced fungicidal action presented in this study, only short-term incubations were carried out. As plotted in Fig. 1, the kinetics of cell growth in YPD medium at 37°C for one day measured by turbidimetry, were virtually indistinguishable between a C. parapsilosis parental strain (Cp) and its isogenic null mutant atc1Δ/ntc1Δ. Furthermore, this result also supports that an operative trehalase activity is unnecessary during the active growth cycle on glucose as carbon source (Fig. 1).
Effect of homozygous disruption of the two genes encoding trehalase activity in C. parapsilosis on cell susceptibility to Itraconazole (ITC) and Voriconazole (VRC). A pre-inoculum of both the parental strain of C. parapsilosis (Cp) and the trehalase-deficient null mutant atc1Δ/ntc1Δ were cultured overnight in YPD medium, diluted to an OD600 nm of 0.3 in the same medium and further incubated at 37°C. Cellular growth was measured turbidimetrically by recording the changes in OD600 nm during 24 h. Symbols: (■) Control, (▲) ITC (0.3 µg/ml), (○) VRC (0.3 µg/ml), (▼) (ITC + rotenone)
It has previously shown that treatment with Itraconazole (ITC) induced a significant toxic action on YPD-grown exponential cells of C. parapsilosis, recorded both as a decrease in the percentage of cell viability and the macroscopic formation of colonies (Sánchez-Fresneda et al. 2022). Indeed, the addition of 0.3 µg/ml ITC (equivalent 2 × MIC) also caused a prominent time-course reduction of the optical density (Fig. 1). Alternative inclusion of Voriconazole (0.3 µg/ml), another recently commercialized azole, gave rise to a similar phenotype (Fig. 1), suggesting that several azoles share a similar pattern of toxicity against C. parapsilosis. Although no experiments were followed beyond 24 h here, exposed cells were able to resume active growth after that time, since these compounds are essentially fungistatic (Sánchez-Fresneda et al. 2022).
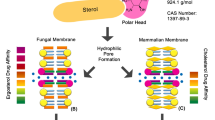
Apart from the well-established inhibition of ergosterol biosynthesis, consistent evidence points to the generation of inner oxidative stress as a contributory factor to the antimycotic action exerted by some azoles against relevant pathogenic fungi (Belenky et al. 2013; Shekova et al. 2017), as has previously been demonstrated with polyenes (Mesa-Arango et al. 2014). Therefore, we evaluated the hypothetical involvement of mitochondrial activity in the C. parapsilosis cell damage caused by exposure to Itraconazole, measured in terms of cell survival. For this purpose, an equivalent number of active cells from the two strains under study were pre-treated with rotenone (0.156 mM) for 1 h at 37°C. This subtoxic concentration is able to effectively inhibit the mitochondrial Complex I without any impairment of cell viability (Fig. 1; Mesa-Arango et al. 2014; Guirao-Abad et al. 2020). Then, 0.3 µg/ml ITC was added and the cultures were further incubated for 1 h or 10 h. As can be seen at Fig. 2 (A and B), the fungicidal activity of this azole was virtually absent after 1 h of treatment. However, longer incubation (10 h) led to Itraconazole having a strong toxic effect of on both C. parapsilosis strains, which could be counteracted by the presence of rotenone (Fig. 2A and B). The reduction in cell viability was also in accordance with the recorded turbidimetric measurements in the absence of rotenone (See Figs. 1 and 2).
Protective action of rotenone and DNP on C. parapsilosis cell viabilitiy against the antifungal toxicity induced by addition of Itraconazole (ITC). YPD-grown exponential cells of the two cell types under study, were preincubated with either rotenone (0.156 mM) or DNP (0.5 mM) for 1 h at 37°C, and immediately treated with ITC (0.3 µg/ml) for 1 h or 10 h A and B or only 10 h C. Identical samples were then harvested, diluted appropriately, and spread on YPD plates. The percentage of cell survival was determined by CFU counting. The data shown are the mean ± SD of three independent experiments. *P < 0.05 and **P < 0.01 represent statistically significant differences with respect to an untreated control according to Mann–Whitney U test. B Tenfold cell suspensions were spotted on YPD plates (5 µl), which were incubated at 37°C and scored after 48 h
Alternative experiments were carried out using the protonophore 2,4-Dinitrophenol (DNP). Notably, this inhibitor of oxidative phosphorylation induces resistance to Fluconazole in Saccharomyces cerevisiae (Kontoyiannis 2000). For comparative purposes, these results are presented in Fig. 2C, but only an assay after 10 h of exposure was processed, since ITC has no effect after 1 h (Figs. 2A and B). As can be seen, 0.5 mM DNP did not affect the percentage of cell viability whether in parental or in atc1Δ/ntc1Δ cells. However, preincubation with 0.5 mM DNP for 1 h partially counteracted the ITC-induced fungicidal action (Fig. 2C), although to a minor extent to that recorded with rotenone (Fig. 2). Furthermore, the atc1Δ/ntc1Δ null mutant always displayed higher susceptibility to ITC respect to the parental strain (Fig. 2; Sánchez-Fresneda et al. 2022). Hence, these results suggest that the antifungal effect triggered by ITC on C. parapsilosis is, at least in part, dependent on a functional mitochondrial activity.
The Itraconazole effect takes place simultaneously with intracellular ROS formation
To corroborate this hypothesis, the level of intracellular ROS formation was measured simultaneously, since indirect data suggest that ROS generation takes place upon azole addition in C. albicans and Aspergillus sp. (Belenky et al. 2013; Shekova et al. 2017). In eukaryotic organisms, mitochondria are the main generators of endogenous ROS, which are formed as by-products of the electron respiratory chain. To check whether this mechanism is operative in the two cell types of C. parapsilosis following ITC exposure, the production of intracellular ROS was determined by flow cytometry using the highly specific staining with DHF (see Methods, Sangalli-Leite et al. 2011).
In these experiments, a positive oxidative stress control (H2O2 50 mM) was run in parallel. The results from a representative assay are shown in Fig. 3. As it has been previously reported (Sánchez-Fresneda et al. 2022) the atc1Δ/ntc1Δ mutant produced a lower basal level of endogenous ROS (Fig. 3). However, the fraction of cells able to generate intracellular ROS after ITC addition was higher in this trehalase-deficient mutant compared with its isogenic parental strain (Fig. 3; central panel). Notably, preincubation with rotenone led to a significant reduction in the capacity to produce endogenous ROS, which was rather similar in both strains, although the inhibitory action of rotenone seemed to be lower in control Cp cells (Fig. 3). This result might be due to the greater ITC-induced ROS production in atc1Δ/ntc1Δ cells (Fig. 3). Hence, we preliminary conclude that ITC can induce a significant oxidative stress through intramitochondrial ROS formation in C. parapsilosis.
Level of intracellular ROS production after treatment with Itraconazol and protective role of rotenone in C. parapsilosis. Equivalent aliquots of YPD-grown exponential cultures of the two strains were incubated for 1 h in the absence or presence of rotenone (0.156 mM) at 37°C, followed by 1 h of treatment with ITC (0.3 µg/ml). ROS were quantified by flow cytometry using DHF in Cp (upper row histograms) and atc1Δ/ntc1Δ (lower row histograms) strains. The histograms display the cell number with respect to the green fluorescence intensity (FL1). A positive marker for acute oxidative stress (50 mM H2O2) was also introduced
Antioxidant enzymatic activities in response to Itraconazole addition
The detection of internal oxidative stress in yeasts is rapidly replied by mounting a general antioxidant response, which involves the activation of enzymatic activities with a detoxifying role (Nikolau et al. 2009). Here, we measured the activity of catalase and superoxide dismutase (SOD) after ITC addition. As shown in Fig. 4A, catalase activity underwent a clear decrease after exposure to ITC compared with untreated controls in parental cells, a diminution that was lower in the atc1Δ/ntc1Δ mutant. Notably, pretreatment with rotenone during 1 h before ITC supply induced a partial recovery of catalase in Cp cells, while in trehalase-deficient cells this antioxidant activity was even slightly higher respect to control samples (Fig. 4A). Similar results were obtained when VRC was introduced instead of ITC (Fig. 4A). In turn, the changes recorded in SOD activity, with or without rotenone exposure, were virtually imperceptible, although the trend of a somewhat larger increase in homozygous atc1Δ/ntc1Δ cells was maintained (Fig. 4B), suggesting that this enzyme may play a minor role in counteracting the ITC-induced inner oxidative stress in C. parapsilosis.
Effect of rotenone on the ITC-induced changes in enzymatic activity shown by the antioxidant enzymes catalase (A) and superoxide dismutase (SOD) (B) in C. parapsilosis Cp and atc1Δ/ntc1Δ strains. Exponential cultures grown in YPD were subjected to the indicated ITC treatment for 1 h with or without preincubation with rotenone. A sample treated with Voriconazole (VRC) was also included. The data shown are the mean ± SD of three independent experiments. *P < 0.05, represents statistically significant differences with respect to an untreated control according to Mann–Whitney U test or between the samples indicated with the brackets
Discussion
The incorporation during recent decades of new triazolic compounds (Voriconazole, Posaconazole or Ravuconazole) together with the availability of classical azoles (Imidazoles, Fluconazole or Itraconazole) has augmented the limited array of antifungal molecules that can be applied in clinical practice against disseminated infections caused by highly prevalent fungi, even though the antifungal action of azoles is essentially fungistatic rather than fungicide (Campoy and Adrio 2017). More importantly, in addition to the search for new, powerful and safer antimycotic substances, an alternative experimental approach has focused on detailed studies of the mechanism(s) of action evolved by the main clinical antifungals, about which our knowledge is still incomplete (Denning and Bromeley 2015). Thus, the generation of an internal oxidative stress through the formation of ROS is a universal action mechanism of the polyene Amphotericin B (González-Párraga et al. 2011; Mesa-Arango et al. 2014) apart from its well-established binding to membrane ergosterol and subsequent pore formation (Brajtburg et al. 1990; Kontoyiannis and Lewis 2002). However, in the case of azoles preliminary evidence points to the involvement of ROS formation as pathogenic factor besides the main inhibition of ergosterol biosynthesis in Aspergillus and Candida (Sangalli Little et al. 2011, Belenky et al. 2013, Shekova et al. 2017). Conversely, Micafungin (an echinocandin) is able to stimulate the immune response and the activation of human macrophages, probably through the exposure of β-glucans on the cell wall surface, without the participation of mitochondrial activity (Guirao-Abad et al. 2018).
Regarding the antifungal treatments directed at the pathogenic yeast C. parapsilosis, no standard protocol has been defined yet, and therapies vary as a function of the geographic areas and the outbreaks examined (Toth et al. 2019). As a guide, C. parapsilosis shows moderate resistance to echinocandins and some clinical isolates are refractory to azoles. We have reported a certain toxic effect of Itraconazole (ITC) on C. parapsilosis, which was increased by disruption of the two genes encoding trehalase activities (Sánchez-Fresneda et al. 2022). To verify the putative role of mitochondrial activity as a recently unveiled mechanism involved in the antifungal action of ITC on C. parapsilosis, we blocked the first step of the electron transport chain through the specific inhibition of Complex I with subtoxic concentrations of rotenone. According to our results, this approach induced a marked protective effect on cell viability (Fig. 2A and B) and, simultaneously, an important reduction in intracellular ROS production (Fig. 3), although the measurements of antioxidant enzymatic activities (catalase and SOD) were less conclusive (Fig. 4). Alternatively, preincubation with the protonophore DNP, which is an inhibitor of the oxidative phosphorylation, also counteracted the toxic effect of ITC, but to a minor extent (Fig. 2C). Taken together, our data strongly support a main role of mitochondrial activity in the toxic action caused by the addition of ITC on C. parapsilosis. It should be noted that, at present, this proposal cannot be extended to other azoles currently used in antifungal therapy. In fact, our previous analysis with the exposure to rotenone plus Fluconazole provided no successful results (Zhang et al. 2015; Sánchez-Fresneda et al. 2022), although DNP is able to increase the resistance to Fluconazole (Kontoyannis 2000), whereas indirect evidence from the use of Voriconazole, obtained by determination of both cell viability and antioxidant enzymatic activities seems more supportive with the involvement of mitochondrial activity (Figs. 1 and 4).
On the other hand, our contribution also reinforces the accomplishment of new research efforts on the central nutritional and physiological pathways in pathogenic fungi as promising therapeutic targets, because some of them are directly involved in the initial infection and further colonization of specific niches inside the host (Prieto et al. 2014; Correia et al. 2019; Wijnants et al. 2020; 2022). Thus, in C. albicans, it has long been known that mutants unable to develop true hypha are avirulent, suggesting that formation of mycelial structures is required for a productive tissue invasion (Lo et al. 1997). Furthermore, in the glycolytic pathway, sugar phosphorylation appears to control the virulence in mice (Wijnants et al. 2020), whereas a regulated depletion of fructose-1,6-bisphosphatase, a key enzyme also involved in gluconeogenesis, hampers the growth and capacity of infection in another mouse model (Rodeki et al. 2006). It seems reasonable, therefore, to propose that manipulation of bottleneck enzymes as well as the design of potent and safe inhibitors against crucial enzymatic steps of the central pathways may be regarded as a valuable way toward attaining efficient antifungals for use in clinical practice.
Data availability
The completed set of data or minor details presented in this study are available on request from the corresponding author. A publicly accessible repository has not been created.
Abbreviations
- AmB:
-
Amphotericin B
- ITC:
-
Itraconazole
- VRC:
-
Voriconazole
- DNP:
-
2,4-Dinitrophenol
- MICs:
-
Minimum Inhibitory Concentration
- PBS:
-
Phosphate Buffer Saline
- ROS:
-
Reactive Oxygen Species
- SOD:
-
Superoxide Dismutase
References
Argüelles JC (2017) Trehalose as antifungal target: the picture is still incomplete. Virulence 8:237–238
Belenky P, Camacho D, Collins JJ (2013) Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep 3:350–358
Brajtburg J, Powderly WG, Kobayashi GS, Medoff G (1990) Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother 34:183–188
Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC (2012) Hidden killers: human fungal infections. Sci Transl Med. 4:165rv13
Campoy S, Adrio JL (2017) Antifungals. Biochem Pharmacol 133:86–96
Cantón E, Pemán J, Quindós G, Eraso E, Miranda-Zapico I, Álvarez M, Merino P et al (2011) Prospective multicenter study of the epidemiology, molecular identification, and antifungal susceptibility of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis isolated from patients with candidemia. Antimicrob Agents Chemother 55:5590–5596
Correia I, Prieto D, Román E, Wilson D, Hube B, Alonso-Monge R, Pla J (2019) Cooperative role of MAPK pathways in the interaction of Candida albicans with the host epithelium. Microorganisms 8:48
Denning DW, Bromeley MJ (2015) Infectious disease: how to bolster the antifungal pipeline. Science 347:1414–1416
Van Dijck P, De Rop L, Szlufcik K, Van Ael E, Thevelein JM (2002) Disruption of the Candida albicans TPS2 gene encoding trehalose-6-phosphate phosphatase decreases infectivity without affecting hypha formation. Infect Immun 70:1772–1782
Garre E, Matallana E (2009) The three trehalases Nth1p, Nth2p and Ath1p participate in the mobilization of intracellular trehalose required for recovery from saline stress in Saccharomyces cerevisiae. Microbiology 155:3092–3099
González-Párraga P, Sánchez-Fresneda R, Zaragoza O, Argüelles JC (2011) Amphotericin B induces trehalose synthesis and simultaneously activates an antioxidant enzymatic response in Candida albicans. Biochim Biophys Acta 1810:777–783
Guirao-Abad JP, Sánchez-Fresneda R, Machado F, Argüelles JC, Martínez-Esparza M (2018) Micafungin enhances the human macrophage response to Candida albicans through β-Glucan exposure. Antimicrob Agents Chemother 62:e02161-e2217
Guirao-Abad JP, Sánchez-Fresneda R, Román E, Plá J, Argüelles JC, Alonso-Monge R (2020) The MAPK Hog1 mediates the response to amphotericin B in Candida albicans. Fungal Genet Biol 136:103302
Holland LM, Schroder MS, Turner SA, Taff H, Andes D, Grozer Z, Gacser A et al (2014) Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog 10:e1004365
Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D (2020) Fungal infections in humans: the silent crisis. Microbial Cell 7:143–145
Kontoyiannis DP (2000) Modulation of Fluconazole sensitivity by the interaction of mitochondria and Erg3p in Saccharomyces cerevisiae. J Antimicrob Chemother 46:191–197
Kontoyiannis DP, Lewis RE (2002) Antifungal drug resistance of pathogenic fungi. Lancet 359:1135–1144
Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR (1997) Nonfilamentous Candida albicans mutants are avirulent. Cell 90:939–949
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mesa-Arango AC, Trevijano-Contador N, Román E, Sánchez-Fresneda R, Casas C et al (2014) The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob Agents Chemother 58:6627–6638
Nikolaou E, Agrafioti I, Stumpf M, Quinn J, Stansfield I, Brown AJ (2009) Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol Biol 9:44
Pál S, Toth R, Nosanchuk J, Cacsaba A, Vagvölgyi C, Nemeth T, Gácser A (2021) A Candida parapsilosis overexpression collection reveals genes required for pathogenesis. J Fungi 7:97
Pedreño Y, Gimeno-Alcañiz JV, Matallana E, Argüelles JC (2002) Response to oxidative stress caused by H2O2 in Saccharomyces cerevisiae mutants deficient in trehalase genes. Arch Microbiol 177:494–499
Perfect JR, Tenor JL, Miao Y, Brennan RG (2017) Trehalose pathway as an antifungal target. Virulence 8:143–149
Pfaller MA, Diekema DJ (2010) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53
Pfaller MA, Andes DR, Diekema DJ (2014) Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the prospective antifungal therapy (PATH) registry 2004–2008. PLoS ONE 97:e101510
Prieto D, Román E, Correia I, Pla J (2014) The HOG pathway is critical for the colonization of the mouse gastrointestinal tract by Candida albicans. PLoS ONE 9:e87128
Rodaki A, Young T, Brown AJP (2006) Effects of depleting the essential central metabolic enzyme fructose-1,6-bisphosphate aldolase on the growth and viability of Candida albicans: Implications for antifungal drug target discovery. Eukaryot Cell 5:1371–1377
Saliba F, Dupont B (2008) Renal impairment and amphotericin B formulations in patients with invasive fungal infections. Med Mycol 46:97–112
Sánchez-Fresneda R, Guirao-Abad JP, Martinez-Esparza M, Maicas S, Valentín E et al (2015) Homozygous deletion of ATC1 and NTC1 genes in Candida parapsilosis abolishes trehalase activity and affects cell growth, sugar metabolism, stress resistance, infectivity and biofilm formation. Fungal Genet Biol 85:45–57
Sánchez-Fresneda R, Muñoz-Megías ML, Yagüe G, Solano F, Maicas S, Argüelles JC (2022) Lack of functional trehalase activity in Candida parapsilosis increases susceptibility to itraconazoles. J Fungi 8:371
Sangalli-Leite F, Scorzoni L, Mesa-Arango AC, Casas C, Herrero E et al (2011) Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect 13:457–467
Shekhova E, Kniemeyer O, Brakhage AA (2017) Induction of mitochondrial reactive oxygen species production by itraconazole, terbinafine and amphotericin B as a mode of action against Aspergillus fumigatus. Antimicrob Agents Chemother 61:e00978-e1017
Thammahong A, Puttikamonkul S, Perfect JR et al (2017) Central role of the trehalose biosynthesis pathway in the pathogenesis of human fungal infections: opportunities and challenges for therapeutic development. Microbiol Mol Biol Rev 81:e00053-e116
Toth R, Nosek J, Mora-Montes H, Gabaldón T, Bliss J, Nosanchuck J, Turner SB, Butler G et al (2019) Candida parapsilosis: from genes to bedside. Clin Microbiol Rev 32:e00111-e118
Tournu H, Fiori P, Van Dijck P (2013) Relevance of trehalose in pathogenicity: some general rules, yet many exceptions. PLoS Pathog 9:e1003447
Trofa D, Gacser A, Noshanchuk J (2008) Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev 21:606–625
Wijnants S, Riedelberger M, Penninger P, Kuchler K, Van Dijck P (2020) Sugar phosphorylation controls carbon source utilization and virulence of Candida albicans. Front Microbiol 11:1274
Wijnants S, Vreys J, Van Dijck P (2022) Interesting antifungal drug targets in the central metabolism of Candida albicans. Trends Pharmacol Sci 43:69–79
Zhang L, Xiao M, Watts MR, Wang H, Fan X, Kong F, Xu YC (2015) Development of fluconazole resistance in a series of Candida parapsilosis isolates from a persistent candidemia patient with prolonged antifungal therapy. BMC Infect Dis 15:340
Acknowledgements
We are indebted to the financial contract provided by PREZERO, Servicios Públicos de Murcia, S.A. (Murcia, Spain). Ethical Approval was not required. We also thank Dr. G. Yagüe (Hospital “Virgen de la Arrixaca”, Murcia) for her kind gift of Itraconazole and Voriconazole.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The authors received no financial support for the research, authorship and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experimental research: FS, MM-E, JCA. Performed the experiments: LM-M, RS-F, MM-E. Analyzed the data: FS, SM, MM-E, JCA. Contributed reagents/materials/analysis tools: FS, SM, MM-E, JCA. Prepared the figures: LM-M, RS-F, MM-E. Wrote the paper: FS, JCA. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muñoz-Megías, M.L., Sánchez-Fresneda, R., Solano, F. et al. The antifungal effect induced by itraconazole in Candida parapsilosis largely depends on the oxidative stress generated at the mitochondria. Curr Genet 69, 165–173 (2023). https://doi.org/10.1007/s00294-023-01269-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-023-01269-z