Abstract
Chalicotheres are a peculiar group of large herbivorous mammals, closely related to extant tapirs, rhinoceroses, and horses, but with large claws instead of hooves. The family Chalicotheriidae consists of two subfamilies, the Schizotheriinae and the Chalicotheriinae. Herein we present chalicothere remains from the Upper Miocene locality of Pogana 1 in Romania, identifying the schizotheriine Ancylotherium pentelicum and an indeterminate chalicotheriine that were both found in the same stratigraphic layer. Thus, the Pogana 1 locality represents one of the very few confirmed cases of the co-occurrence of the two subfamilies within one fossiliferous horizon in the same fossil site. A detailed review of all localities where the two subfamilies have been reported to co-occur shows that this is a rare phenomenon that is almost exclusively observed in the Turolian of the Balkan-Iranian zoogeographical province. This is probably due to provincial differences in the palaeoenvironment. The data presented here support the hypothesis of a diverse mosaic environment in the Balkan-Iranian province with both closed environments and open habitats that was able to sustain a rich and diverse large mammal fauna.
Similar content being viewed by others
Introduction
Chalicotheres first appeared in the Eocene and survived until the Pleistocene (Coombs 1989; Coombs and Cote 2010). In the Neogene, they reached their highest diversification and geographical distribution, with representatives of this group being found in North America, all over Eurasia, and Africa. During this time frame, they were especially successful in Eurasia, with the presence of both subfamilies, the Schizotheriinae and the Chalicotheriinae (Heissig 1999; Chen 2008). The Schizotheriinae first appeared in the Oligocene in Asia and North America (Coombs 1989; Li et al. 2022), whereas the first Chalicotheriinae are known from the Early Miocene of Asia and Africa (Coombs 1989; Coombs and Cote 2010).
In North America, only one of the two subfamilies is known, the Schizotheriinae. In Africa, both subfamilies occur, but they are separated by an approximately 4 million-year long time gap (Coombs 1989; Coombs and Cote 2010). Only in Eurasia, both subfamilies existed in the same time frame of over 20 million years (Early Miocene to Early Pleistocene), though there are only few localities where both have been reported together (Coombs 1989).
The potential coexistence of the two subfamilies, Schizotheriinae and Chalicotheriinae, has been a matter of discussion for many years. Some researchers have even argued that they were not able to coexist in the same environment due to their potentially similar ecology and that the few reports of coexistence are due to some kind of sampling bias (Bonis et al. 1995). Others have supported the idea that the reported cases of co-occurrence are valid and suggested that their ability to coexist is related to differences in their preferred habitats and diets (Coombs 1989; Koufos 2012). Chalicotheriinae have lower crowned teeth and much longer forelimbs compared to their hindlimbs and are traditionally seen as forest-dwellers (Zapfe 1979; Coombs 1989). Schizotheriinae on the other hand, have higher crowned teeth and the difference in their limb proportions is not as prominent, with proportions similar to those of extant okapis, which might have enabled them to also survive in more open or patchy habitats (Schaub 1943; Coombs 1983; Coombs and Cote 2010). These differences in their anatomy have led to the idea of different preferred habitats that affected their potential coexistence (Coombs 1989; Giaourtsakis and Koufos 2009; Koufos 2012).
In this study, we describe new chalicothere material from the Upper Miocene locality Pogana 1 (Romania), which yields both a schizotheriine and a chalicotheriine from the same horizon. Thus, this locality is one of the few cases where the coexistence of both subfamilies can be verified. Additionally, the palaeobiogeography of both subfamilies, together with a review of all localities with occurrences of both subfamilies is included here and their implications for the palaeoenvironmental reconstruction of the Balkan-Iranian province are discussed.
Geological setting
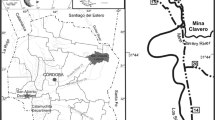
The Upper Miocene locality of Pogana 1 (Romania) has yielded three specimens of chalicotheres, which were all found in the same layer (Fig. 1), within the open sand pit located 15 km north of Bârlad town and south of Pogana village (Vaslui County), on the western slope of Vii Hill (‘Dealul Vii’) (Ursachi 2016).
The sedimentary deposits exposed in the Pogana area (Romania) belong to the Scythian Platform (Săndulescu 1984) and, more specifically, to the westernmost area of this platform, the so-called “Bârlad Platform” (sensu Ionesi 1994). Here, only deposits of Khersonian – Maeotian age crop out.
The Maeotian succession includes the volcanic cinerites of Nuţasca-Ruseni, as shown by Jeanrenaud (1971). These layers are excellent marker horizons that can be easily identified in the field, in both the deltaic and brackish facies areas of the Maeotian deposits. The Maeotian sediments in the region were mainly deposited in a fluvial environment. The lower part of the Maeotian succession is composed of brown andesitic tuffs in paragenesis with cinerites, which are gradually replaced by yellow greenish cineritic/tuffic sands. So far, three cineritic layers have been described. The upper part of the Maeotian deposits comprises complex alternations of fine yellow sands, which are poorly sorted, as well as layers of clays and silts. Some sands show intercalations of lenticular sandstones which reach a diameter of 2.5 m, as identified on the Studineț and Tutova Valleys (near the Perieni locality). The upper member also includes some sandy layers with accumulations of the bivalve Unio wetzleri.
The open sand pit Pogana 1, is situated well above the cineritic sands and sandstones. The geological section of Pogana 1 comprises a basal yellow sand of 2 m thickness, covered by sands with trough cross-bedding and convolute beddings. These sands are covered unconformably by coarse sands with angular mud-clasts with extensive cross-stratification, followed by yellow sands with ripple-cross lamination and horizontal bedding covered by a green mudstone. The vertebrate remains, including the herein studied specimens, were collected from these lens-like deposits in the coarse sands (Fig. 1).
Institutional abbreviations:
MVP-SN-PG, Vasile Pârvan Museum, Natural Sciences Branch, Bârlad, Romania; PG, Pogana locality; NHMW, Naturhistorisches Museum in Vienna, Austria.
Systematic paleontology
Mammalia Linnaeus, 1758
Perissodactyla Owen, 1848
Chalicotheriidae Gill, 1872
Schizotheriinae Holland & Peteron, 1914
Ancylotherium Gaudry, 1863
Ancylotherium pentelicum (Gaudry & Lartet, 1856)
(Fig. 2)
Proximal phalanx of Ancylotherium pentelicum (MVP-SN-PG-63) from the Late Miocene of Pogana in Romania, in dorsal (a–b), ventral (c–d), left lateral (e–f), and right lateral (g–h) views. Abbreviation: α, angle formed by the proximal articular facet and the long axis of the shaft. Scale bar equals 5 cm
Referred material: MVP-SN-PG-63, proximal phalanx of the pes.
Description
Specimen MVP-SN-PG-63 is a well-preserved proximal phalanx. The surface of the bone is somewhat damaged. The bone belonged to an adult individual as is demonstrated by the fully fused proximal epiphysis. It is not possible to determine the exact digit to which this phalanx belonged. The proximal articular facet for the metapodial is ovally heart-shaped, wider than long, and rather shallow, with a small incision in the middle of its proximo-ventral border. It is large and placed obliquely to the proximo-distal axis of the phalanx, towards the dorsal side of the bone, forming an acute angle (α in Fig. 2f). The ventral side of the bone bears well-developed tuberosities in its proximal part that act as areas for muscle attachments. Distally the phalanx slightly narrows. The distal articular facet for the middle phalanx is placed obliquely, almost entirely towards the ventral side of the bone, forming an acute angle to the proximo-distal axis of the bone. The bilateral keels of the distal articulation are slightly asymmetrical.
Comparison
Specimen MVP-SN-PG-63 represents a typical proximal phalanx of a chalicotheriid. Its proximal articular facet for the metapodial is proximo-dorsal oriented, whereas the distal facet for the middle phalanx is placed more ventrally. It differs from proximal phalanges of chalicotheriines, like Anisodon grande, in being somewhat thicker dorsoventral (Fig. 3a, d) and having a more obliquely placed proximal articular facet. The morphology of MVP-SN-PG-63 differs from phalanges of the schizotheriine Metaschizotherium fraasi in having a relatively narrower proximal epiphysis (Fahlke and Coombs 2009). It is much more similar to phalanges of Ancylotherium pentelicum, where the proximal articular facet is also more obliquely placed (Fig. 3a-c) and the proximal phalanx is in general less flattened (Roussiakis and Theodorou 2001). Ancylotherium pentelicum is well known from the Upper Miocene deposits of the Eastern Mediterranean (Giaourtsakis and Koufos 2009; Kampouridis et al. 2022) and its presence in Pogana 1 has already been confirmed by Codrea et al. (2019). Nonetheless, thus far, no proximal phalanx of the pes belonging to Ancylotherium pentelicum has been described. Roussiakis and Theodorou (2001), while observing some morphological and metrical differences in their sample of 10 proximal phalanges from Pikermi and Halmyropotamos, were unable to differentiate between phalanges of the manus and the pes. Schaub (1943) described the forelimb of Ancylotherium pentelicum in much detail, including proximal phalanges from the Late Miocene localities of Pikermi and Samos in Greece. Based on these descriptions it was possible to identify four proximal phalanges from Pikermi housed in the Natural History Museum in Vienna, Austria (NHMW) as belonging to the pes. The proximal phalanx of the second digit of the manus fuses with the middle phalanx and forms the duplex bone (Schaub 1943; Coombs and Rothschild 1999; Roussiakis and Theodorou 2001) in contrast to the phalanges of the pes, which do not fuse. The proximal phalanges of the third and fourth digits of the manus of Ancylotherium pentelicum are relatively similar and are generally larger, more elongated, and have less developed tuberosities in the proximal part on their ventral side than the proximal phalanges of the pes in Ancylotherium pentelicum.
Morphological comparison of proximal phalanges of Chalicotheriidae. a. proximal phalanx of the pes of Ancylotherium pentelicum (MVP-SN-PG-63) from the Upper Miocene of Pogana 1 in Romania, in left lateral view. b. proximal phalanx of the pes of Ancylotherium pentelicum (NHMW 2019/0098/0013) from the Upper Miocene of Pikermi in Greece, in left lateral view. c. proximal phalanx of digit III of the manus of Ancylotherium pentelicum (NHMW 2019/0098/0016, cast) from the Upper Miocene of Pikermi in Greece, in right lateral view (mirrored). d. proximal phalanx of digit III of the manus of Anisodon grande from the Middle Miocene of Devínska Nová Ves in Slovakia, in left lateral view (Zapfe 1979: fig. 94). Abbreviation: α, angle formed by the proximal articular facet and the long axis of the shaft. Not to same scale
Based on the metric comparison (Fig. 4) the Pogana 1 specimen is smaller than the proximal phalanges of third and fourth digits of the manus of Ancylotherium pentelicum from the Late Miocene localities Pikermi and Samos (Schaub 1943) and larger than the proximal phalanges of the pes of Anisodon grande from the Middle Miocene locality Devínska Nová Ves (Zapfe 1979). Its dimensions fit perfectly within the size range of the measured proximal phalanges of the pes of Ancylotherium pentelicum from Pikermi (Fig. 4; Table 1). These overlap with the lower part of the size range of the proximal phalanges of the manus of Anisodon grande based on the measurements of Zapfe (1979), but they differ morphologically (Fig. 3). In both MVP-SN-PG-63 and the phalanges of the pes of Ancylotherium pentelicum on the posterior side the tuberosities in the proximal part are very large, whereas in the phalanges of the manus of Anisodon grande they are not as prominent, also the angle between the proximal articular facet and the shaft of the bone is smaller in MVP-SN-PG-63 and the phalanges of the pes of Ancylotherium pentelicum than in the phalanges of the manus of Anisodon grande (Fig. 3). Therefore, MVP-SN-PG-63 can be assigned to Ancylotherium pentelicum. It should be taken into account that Ancylotherium pentelicum has been suggested to exhibit a strong sexual size dimorphism (Kampouridis et al. 2022). Although the presence of size dimorphism in the phalanges has not been confirmed yet and might prove difficult, this complicates the identification of the exact digit within the manus or pes of an isolated phalanx. Nonetheless, based on its small size (maximal length = 66 mm) and distinct morphology, MVP-SN-PG-63 is herein identified as a proximal phalanx of the pes of Ancylotherium pentelicum.
Metric comparison of proximal phalanges of Chalicotheriidae. The black dot represents the proximal phalanx of the pes of Ancylotherium pentelicum (MVP-SN-PG-63) from the Upper Miocene locality Pogana 1 in Romania. Measurements of Ancylotherium pentelicum from Pikermi and Samos in Greece are from Schaub (1943: p. 26) for the manus and original data (Table 1) for the pes. Measurements of Anisodon grande from Devínska Nová Ves in Slovakia are from Zapfe (1979)
Chalicotheriinae Gill, 1872
Chalicotheriinae indet.
(Fig. 5)
Calcaneum of Chalicotheriinae indet. (MVP-SN-PG-64) from the Upper Miocene of Pogana 1 in Romania in dorsal (a–b), ventral (c–d), medial (e–f) and posterior (g–h) view. Abbreviations: β, angle formed by the sustentaculum tali and the long axis of the shaft in ventral view; γ, angle formed by the sustentacular facet and the long axis of the shaft in medial view; cft, calcaneal sustentacular facet; sta, sustentaculum tali; tc, tuber calcis. Gray colour corresponds to damaged areas in the bone. Scale bar equals 5 cm
Referred material: MVP-SN-PG-64, fragmentary left calcaneum.
Description
Specimen MVP-SN-PG-64 is a partial left calcaneum. It is very damaged and missing much of its sustentaculum tali and articular facets for the astragalus, only the calcaneal sustentacular facet remains. The dorsal part of its shaft is also broken, and the tuber calcis is somewhat damaged, though its posteromedial portion is preserved, showing that it did not continue any further. Dorsally, a large sustentacular facet is visible on the sustentaculum tali, for the articulation of the astragalus. This articular facet is anteroposteriorly oriented and almost parallel to the shaft of the calcaneum, forming only a slight angle (γ in Fig. 5). In ventral view, a ridge is visible, that extends from the distal end of the shaft to the lateral edge of the sustentaculum tali. However, both its beginning and end are not preserved, due to damage to the bone. The shaft seems rather straight and widens posteriorly towards the tuber calcis. The ventral side of the shaft features a relatively rugose surface for muscle attachments. The shaft and the sustentaculum tali form an acute, almost right angle.
Comparison
Specimen MVP-SN-PG-64 is rather badly preserved, but some comments about its taxonomic attribution are possible. The comparisons (see Fig. 6) are based mainly on the descriptions and illustrations of the chalicotheriine Anisodon grande from the Middle Miocene of Devínska Nová Ves (Slowakia) made by Zapfe (1979). Additionally, a chalicotheriine calcaneum from the Late Miocene (Vallesian) of Charmoille (Switzerland) originally described as Chalicotherium goldfussi by Schaefer and Zapfe (1971) is used. The identification of this specimen was mainly based on its contemporaneous age to the type locality of Chalicotherium goldfussi, Eppelsheim (Germany). However, there is no calcaneum of this species known from its type locality, therefore we refer to this specimen as "Chalicotherium goldfussi". Lastly, the comparison includes the calcaneum of the schizotheriine Ancylotherium pentelicum from the Late Miocene (Turolian) of Pikermi (Greece).
Morphological comparison of calcanea of Chalicotheriidae. Chalicotheriinae indet. (MVP-SN-PG-64) from the Upper Miocene of Pogana 1 in Romania (a–b). “Chalicotherium goldfussi” from the Upper Miocene of Charmoille in Switzerland (Zapfe 1979) (c–d). Anisodon grande from the Middle Miocene of Devínska Nová Ves in Slovakia (Zapfe 1979) (e–f). Ancylotherium pentelicum from the Upper Miocene of Pikermi in Greece (Zapfe 1979) (g–h). Each specimen is presented in dorsal (upper row) and ventral (lower row) view. Not to same scale
The calcaneal sustentacular facet of the Pogana 1 specimen is oriented almost parallel to the shaft of the calcaneum as in the Chalicotheriinae Anisodon grande from Devínska Nová Ves (Slovakia) and “Chalicotherium goldfussi” from Charmoille (Switzerland), whereas in the schizotheriine Ancylotherium pentelicum from Pikermi (Greece), it is almost horizontal (Zapfe 1979; Roussiakis and Theodorou 2001). In the Pogana 1 specimen, the sustentaculum tali, although its tip is broken, seems to form a slightly acute angle (β in Fig. 6b), more similar to those in the chalicotheriines “Chalicotherium goldfussi” (β in Fig. 6d) and Anisodon grande (β in Fig. 6F), whereas in the schizotheriine Ancylotherium pentelicum, this angle is obtuse (β in Fig. 6h). Thus, specimen MVP-SN-PG-64 can be assigned to a member of the Chalicotheriinae.
MVP-SN-PG-64 seems to differ from both Chalicotherium goldfussi and Anisodon grande, the two best-known representatives of this group during the Miocene in Europe. Anisodon grande has a narrow and much more elongated shaft and the calcaneal sustentacular facet is smaller. Thus, the Pogana 1 specimen does not belong to Anisodon grande. The “Chalicotherium goldfussi” calcaneum from Charmoille has a wider shaft and a bigger sustentacular facet, more similar to the Pogana 1 specimen. Though, specimen MVP-SN-PG-64 differs from the calcaneum of “Chalicotherium goldfussi” from Charmoille in having a prominent ridge on the ventral side of the sustentaculum tali. Moreover, in the calcaneum of “Chalicotherium goldfussi” from Charmoille, the postero-medial edge of the sustentaculum tali is projecting much more posteriorly compared with Anisodon grande; however, this portion is not preserved in the Pogana 1 specimen. Furthermore, for other chalicotheriines from the Turolian of the Balkan peninsula such as Kalimantsia bulgarica (Bulgaria) and Anisodon macedonicus (Greece) the postcranium, including the calcaneum, is completely unknown (Bonis et al. 1995; Geraads et al. 2001, 2006). It is not possible to compare these taxa and identify the specimen any further. Therefore, specimen MVP-SN-PG-64 will be referred to as Chalicotheriinae indet.
Discussion
Coexistence of the Chalicotheriinae and Schizotheriinae has been documented only in a few localities in Eurasia (Coombs 1989). In North America, the geologically oldest chalicothere remains are known from the Eocene and belong to taxa like Eomoropus, with the first Schizotheriinae arriving in the Miocene (Coombs 1989). Schizotheriinae are well documented in the Neogene deposits of North America, with the genera Moropus and Tylocephalonyx having been studied in detail (Holland and Peterson 1914; Coombs 1978a, 1979). Chalicotheriinae have never been reported and probably never made it into the New World (Coombs 1989; Coombs and Cote 2010).
In Africa, chalicotheriines first appeared in the Early Miocene with Winamia (Coombs and Cote 2010; Pickford 2020; Handa et al. 2021). They existed there until the Middle Miocene (Coombs and Cote 2010). The Schizotheriinae first appeared in the Late Miocene and are documented until the Pleistocene (Coombs and Cote 2010).
Asia has been considered the centre for the diversification of the chalicotheres, with three genera described from the Eocene, Eomoropus, Grangeria, and Litolophus (Coombs 1989; Bai 2008; Bai et al. 2010). The first representative of the Chalicotheriidae appeared during the Oligocene in Eurasia with the schizotheriine genus Schizotherium (Coombs 1978b). During the Early Miocene, the Chalicotheriinae first appeared in Asia with the species “Chalicotherium” pilgrimi (Coombs 1989). Both subfamilies are well documented during the Late Miocene and finally became extinct in the Pleistocene (Fahlke et al. 2013).
Palaeobiogeographical review
So far, the co-occurrence of members of both subfamilies has only been reported from Eurasia (Coombs 1989). Except for one locality from the Pleistocene of China all localities with both subfamilies present are found in the Neogene of Eurasia (Figs. 7–8). As mentioned by Bonis et al. (1999), however, many of these reports of co-occurrence may represent cases of sampling bias by accidentally mixing material from different localities and/or horizons. This is especially the case for historical collections of fossils that were excavated in the 19th or the beginning of the 20th century, and therefore might not be evidence for the coexistence of the two subfamilies. The Upper Miocene deposits in Pogana 1 (Romania) yield one of the few certain cases, where material of a schizotheriine (Ancylotherium pentelicum) was found along with material of a chalicotheriine (Chalicotheriinae indet.) in the same fossiliferous horizon. To highlight the importance of this record we provide a review of all published cases of co-occurrence of the two groups and reassess the available data in order to identify the localities where the coexistence of the two subfamilies is certain.
Map with the fossiliferous localities at which it has been suggested that both Schizotheriinae and Chalicotheriinae occurred during the Neogene and Quaternary. Empty symbols represent localities that are not considered herein as certain cases for the co-occurrence of the two subfamilies, whereas filled symbols represent localities that are herein considered as certain cases
Early Miocene:
So far, only two potential co-occurrences have been reported from the Early Miocene, both from Asia (Fig. 7). The first possible case is from the Early Miocene of the Lanzhou Basin, where Qiu et al. (1998) reported a schizotheriine mandible, of the new species Phyllotillon huangheensis (now referred to as Borissiakia huangheensis sensu Li et al. 2022), from the locality GL9503. He also described two skulls and a mandible of an indeterminate chalicotheriine from another locality, GL9516. Even though the two taxa were found in the same stratigraphic layer, a white sandstone in the lower part of the Middle Member of the Xianshuihe Formation, they were found at different fossil sites (Qiu et al. 1998) and therefore it remains unclear if the two species actually coexisted.
The second locality is found in the Bugti Hills in Pakistan, where both, the schizotheriine Phyllotillon naricus and the chalicotheriine “Chalicotherium” pilgrimi, have been reported from the Lower Miocene deposits (Colbert 1935; Welcomme et al. 2001; Antoine et al. 2013). But the exact stratigraphic context of these finds is unclear. Nonetheless, two distinct species of chalicotheres are found together in the fossiliferous level 4 (= Q), more specifically in the fossil site Kumbi 4F (Antoine pers. comm.). Thus, Kumbi 4F in the Bugti Hills is the only Early Miocene locality where two distinct chalicotheres are documented together.
Middle Miocene:
During the Middle Miocene, there are four reports of co-occurrences of both subfamilies. At the Middle Miocene locality of La Grive St.-Alban in France one of the earliest occurrences of a chalicotheriine in Europe is found (referred to as Chalicotherium goldfussi by Anquetin et al. 2007). Additionally, a schizotheriine (Koenigswald 1932) is also known from this locality, for which the species Phyllotillon grivensis was erected (Mein and Ginsburg 2002). Though its taxonomic status is still discussed, it could represent the earliest Ancylotherium (see Coombs 2009; Fahlke and Coombs 2009). However, it must be noted that the name La Grive St.-Alban refers to a number of Middle Miocene fissures. The old excavations, the site of which was named “fente A”, have yielded material of both the chalicotheriine and the schizotheriine (Depéret 1892; Koenigswald 1932). However, it is not possible to clarify if the material is truly contemporaneous. Furthermore, Mein and Ginsburg (2002) only identified the schizotheriine Phyllotillon grivensis in their material from La Grive St.-Alban.
Two other Middle Miocene localities that have been reported to yield both subfamilies are Thannhausen and Stätzling in Germany. In both localities, the schizotheriine Metaschizotherium has been found (Metaschizotherium sp. in Thannhausen and Metaschizotherium bavaricum in Stätzling) along with a chalicotheriine, possibly Anisodon (Fahlke and Coombs 2009). Both localities are found in the Upper Freshwater Molasse in Southern Germany. The fossils from Thannhausen were collected by Georg Geisselmann, the owner of the sand and gravel pit where the fossils originate from, who noted that the fossils come from different levels, mainly from the base of a fine sand (Dehm 1980). Regarding Stätzling, its main fauna is recovered from the upper sand and gravel layers, while the lower stratigraphic horizons have also yielded fossils (Dehm 1980). It cannot be verified whether the chalicotheriines and the schizotheriines were found in the same horizons. Therefore, their co-occurrence in Thannhausen and Stätzling cannot be considered as certain.
The last Middle Miocene locality that yielded fossils of both subfamilies is Tunggur in China. The chalicotheriine there was described as a new species, Chalicotherium brevirostris (Colbert 1934; Liu and Zhang 2012). However, Tunggur is not a name for a specific fossil site, but characterises a wider area; therefore, it remains unknown whether the schizotheriine and the chalicotheriine were found at the same exact locality and horizon. Thereby, the Middle Miocene does not include any certain case of coexistence of the two subfamilies, due to the lack of stratigraphic information. This highlights the importance of future chalicothere finds from stratigraphically controlled excavations.
Late Miocene:
This is the only sub-epoch with as many as eight reported localities where both subfamilies were found together. All of these fossil sites are located in the Greco-Iranian zoogeographical province (sensu Bonis et al. 1992), herein referred to as the Balkan-Iranian zoogeographical province that includes Southeast Europe, and Anatolia as far as Iran, following Spassov et al. (2018).
Pikermi is one of the most famous localities from the Upper Miocene in Eurasia (Roussiakis et al. 2019). It has been excavated for well over a century (Wagner 1848; Gaudry 1862; Roussiakis et al. 2019). The first excavations took place between the middle of the 19th and the beginning of the twentieth century (Wagner 1848; Gaudry and Lartet 1856; Gaudry 1862). The material collected during this time all came from sediments exposed by the river that runs through the town of Pikermi. Several fossil sites were excavated within the river, but their exact location or stratigraphic correlation is not known. Based on new data, these fossil sites should span the time frame of 7.33 to 7.29 Ma (Böhme et al. 2017). In the 1970s, new excavations were carried out in younger horizons (7.17 Ma; Böhme et al. 2017) very close to the classical Pikermi sites (Symeonidis 1973; Symeonidis et al. 1973), this fossil site is now known as Chomateri. The classical Pikermi area is the type locality of Ancylotherium pentelicum. Despite being well represented in the old collections of Pikermi, Ancylotherium pentelicum has not been reported yet from Chomateri (Symeonidis 1973; Roussiakis and Theodorou 2001; Tsoukala 2022). On the other hand, chalicotheriine material that was originally assigned to Chalicotherium goldfussi has been reported from both the classical locality of Pikermi (Butler 1965), as well as Chomateri (Symeonidis 1973). Nonetheless, the coexistence of these two species in Pikermi is uncertain and, although very likely, it should be treated with caution (Roussiakis and Theodorou 2001). Currently, new excavations are taking place in Pikermi (Theodorou et al. 2010; Roussiakis et al. 2014) which are focussed on the exact stratigraphy and taphonomy of the fossil sites and may solve this question.
In southwestern Bulgaria three localities (Fig. 8) are known with remains of both subfamilies (Geraads et al. 2001, 2006; Boev 2017; Spassov et al. 2019). The first locality, Kalimantsi, includes several fossil sites that are of Turolian age (Spassov et al. 2006; Böhme et al. 2018). One of these, Kalimantsi-Pehtsava, has yielded an almost complete chalicothere skull (K-631), the type of the chalicotheriine Kalimantsia bulgarica (Geraads et al. 2001). At the same fossil site an astragalus (K-608) of the schizotheriine Ancylotherium pentelicum was found (Geraads et al. 2001, 2006). Close to Kalimantsi, the second locality, Hadjidimovo is situated, which is also of Turolian age (Hristova et al. 2003; Böhme et al. 2018). In this area as well, more than one fossil site has been discovered, of which Hadjidimovo-1 is the richest one. Here several postcranial elements assigned to Chalicotherium ? sp. cf. C. goldfussi were found, along with several dental and postcranial elements as well as a partial juvenile skull of Ancylotherium pentelicum (Geraads et al. 2001, 2006). Gorna Sushitsa is the third locality from the Upper Miocene of southwestern Bulgaria. Its stratigraphy was recently re-evaluated (Böhme et al. 2018) and a detailed study of its faunal assemblages was published by Spassov et al. (2019). In total, 14 fossiliferous horizons with vertebrates were identified. Both the schizotheriine Ancylotherium pentelicum and the chalicotheriine Anisodon sp. are known, but they were found in different horizons (Spassov et al. 2019), e.g., Ancylotherium was found at the fossil site GS7, with an age of 7.41 Ma, whereas Anisodon was found at GS4 and GS10, with ages of 7.38 Ma and 7.36 Ma (Spassov et al. 2019). Thus, there is an age difference between the occurrences of the schizotheriine Ancylotherium and the chalicotheriine Anisodon of at least 30,000 years. Despite the fact that this time frame is geologically speaking only a very short time period, there is no definite proof and we do not consider the Gorna Sushitsa locality as evidence for the co-occurrence of both subfamilies.
Map with the fossiliferous localities at which it has been suggested that both Schizotheriinae and Chalicotheriinae occurred together during the Late Miocene. Empty symbols represent localities that are not considered herein as certain cases for the co-occurrence of the two subfamilies, whereas filled symbols represent localities that are herein considered as certain cases
In North Macedonia co-occurrences of both subfamilies have been reported from three localities (Fig. 8). Almost half a century ago, the palaeontologist Risto Garevski studied the Late Miocene ‘Pikermi-fauna’ from the area around Veles in North Macedonia. The localities in this region were lumped together under the name ‘Titov Veles’ (Spassov et al. 2018). Karaslari, is the most diverse vertebrate locality in North Macedonia, with 22 mammalian species (Spassov et al. 2018). Garevski (1974) described a skull of Ancylotherium pentelicum from Karaslari. Additionally, Garevski and Zapfe (1983) described a chalicothere mandible, which they attributed to Chalicotherium goldfussi, from the same locality. Recently, Spassov et al. (2018) re-evaluated the Late Miocene faunal assemblages from North Macedonia and confirmed the existence of Ancylotherium pentelicum in Karaslari, attributing further material to this species, while referring the chalicotheriine mandible to Anisodon sp., following the assignment of Anquetin et al. (2007). The second locality in North Macedonia is Kiro Kuchuk, which has yielded 17 mammalian taxa (Spassov et al. 2018). There is only one third upper molar of an indeterminate chalicotheriine (Spassov et al. 2018), but several specimens of Ancylotherium pentelicum, including upper and lower jaw fragments and a remarkable, articulated hindlimb (Spassov et al. 2018). The third locality, Prevalets, is also situated in the area of Veles in North Macedonia and has also yielded a typical diverse Late Miocene fauna. This fauna was first described by Schlosser (1921), who included Nestoritherium pentelici (today known as Ancylotherium pentelicum) in the faunal list. Spassov et al. (2018) described two associated upper teeth of an indeterminate chalicotheriine from Prevalets that were collected by R. Garevski decades after the first description of Schlosser (1921). Spassov et al. (2018) did not mention any Ancylotherium therefore the occurrence of this taxon in Prevalets is unproven and their co-occurrence is uncertain.
In Turkey, only one locality, Akkaşdaği from the Turolian, is known where both subfamilies are recorded (Fig. 8). Three chalicotheriid specimens have been described from there, a maxilla of a juvenile individual, a duplex bone of the second digit of the manus, and a calcaneum. This material was initially identified as the schizotheriine Ancylotherium pentelicum (Saraç and Sen 2005). However, the morphology of the squared, low-crowned teeth of the juvenile maxilla (AK2-293) from Akkaşdaği differs from that of Ancylotherium pentelicum or other schizotheriines (Fahlke et al. 2013; Spassov et al. 2018). Therefore, it most likely belongs to an anisodont chalicotheriine (sensu Coombs and Göhlich 2019), based on the squared, low-crowned teeth. This maxilla was found in the same bone pocket as the calcaneum (AK2-438) that most definitely belongs to Ancylotherium pentelicum (Saraç and Sen 2005). Thus, Akkaşdaği is the only locality in Anatolia where the co-occurrence of both, a schizotheriine and a chalicotheriine can be confirmed.
Overall, six Upper Miocene fossil sites have definitely yielded material from both subfamilies (Fig. 8). These localities are Pogana 1 (Romania), Kalimantsi-Pehtsava, Hadjidimovo-1 (Bulgaria), Karaslari, Kiro Kuchuk (North Macedonia), and Akkaşdaği (Turkey) (Fig. 8). Interestingly, the schizotheriine taxon is consistently identified as Ancylotherium pentelicum, which is the only schizotheriine taxon from the Turolian of the Balkan-Iranian province. However, the chalicotheriine material is much more difficult to determine, due to its complicated taxonomic history and fragmentary remains and the existence of up to three different taxa has been suggested (see also Bonis et al. 1992; Anquetin et al. 2007).
Pliocene:
After the Late Miocene, chalicotheres became extinct in Europe, but survived in Africa and Asia (Chen 2008; Coombs and Cote 2010). In the Pliocene, there has been only one report of the two subfamilies appearing together, and that is from the locality of Duikang in China (Fig. 7), with the genera Hesperotherium and Ancylotherium (Deng et al. 2011). However, the skull and associated mandible, based on which the chalicotheriine Hesperotherium was identified in Duikang, were later revised by Chen et al. (2012) and assigned to Ancylotherium sp. and therefore only this schizotheriine is present in Duikang.
Pleistocene:
From the Pleistocene only one locality is known that has yielded both chalicothere subfamilies, which is Huangjiawan in the Shaanxi Province in China (Fig. 7). Its fauna is of Early Pleistocene age and has yielded both the schizotheriine Ancylotherium sp., based on a maxilla that preserves the P4–M2, and the chalicotheriine Hesperotherium sinense (also known as Nestoritheriun sinense), based on a maxilla preserving P4–M3 and an almost complete mandible (Li and Deng 2003). Hesperotherium sinense is the most commonly identified chalicothere during this period. On the other hand, Ancylotherium sp. in Huangjiawan is the latest known occurrence of Schizotheriinae in Eurasia. The identification of this specimen as a schizotheriine was also confirmed by Chen et al. (2012), although stating that its taxonomic status is still controversial and the generic attribution is uncertain. Thereby, the Early Pleistocene locality of Huangjiawan is the only post-Neogene locality where both subfamilies have been reported together this far and is thus the youngest case of such a co-occurrence.
Distribution summary:
The review of the co-occurrence of both subfamilies demonstrates that it is a rare phenomenon, which is almost exclusively observed in the Upper Miocene of the Balkan-Iranian province. There is only one case from the Early Miocene, in the Bugti Hills in Pakistan (Colbert 1935), and one from the Early Pleistocene, at the locality Huangjiawan in China (Li and Deng 2003). In the Late Miocene, there is a large disparity in the distribution of the two subfamilies of chalicotheres. During the Vallesian, Chalicotheriinae are found in several localities across Europe, such as Eppelsheim (Germany), Can Llobateres and Vallès Penedès (Spain) (Coombs 1989; Morales et al. 1999), but Schizotheriinae are extremely rare, with only two occurrences of Ancylotherium in Europe, in Los Valles de Fuentidueña in Spain and Pentalophos-1 in Greece (Koufos 2012). On the other hand, during the Turolian Schizotheriinae are almost completely absent in Central and Western Europe, in contrast to Chalicotheriinae (Bonis 1999; Giaourtsakis and Koufos 2009; Fahlke et al. 2013). Similarly, in Eastern Asia Chalicotheriinae seem to be more common than Schizotheriinae (Chen 2008; Chen et al. 2012). The Balkan-Iranian zoogeographical province has yielded a significant number of fossil sites with occurrences of both subfamilies, with the schizotheriine Ancylotherium pentelicum being the most common representative of the family, during the Turolian age. Bonis et al. (1999) argued that both subfamilies were unable to coexist and that the few recorded co-occurrences were doubtful and probably due to the accidental mixing of material from different fossil sites (Geraads et al. 2001). Herein we show that there are six localities where the co-occurrence of both subfamilies can be undisputedly verified: 1, Pogana 1 in Romania, 2, Kalimantsi-Pehtsava, 3, Hadjidimovo-1 in Bulgaria, 4, Karaslari, 5, Kiro Kuchuk in North Macedonia and 6, Akkaşdaği in Turkey (Fig. 8). Notably, almost all of them are found in the western part of the Balkan-Iranian zoogeographical province during the Turolian age (Geraads et al. 2001, 2006; Saraç and Sen 2005; Spassov et al. 2018, 2019). The distribution of chalicotheres is most probably controlled by the environment, which was characterised by open grasslands that were dominated by C3 plants during this time (e.g., Fortelius et al. 2014; Böhme et al. 2017). During this time period, however, the expansion of C4 plants took place, which was mainly driven by a trend towards cooler and more arid climate in the Late Miocene (Tzanova et al. 2015; Herbert et al. 2016; Kaya et al. 2018; Böhme et al. 2021). This, along with other regional changes in the environment most likely affected the potential for coexistence of the two subfamilies. However, it has to be noted that chalicotheres are very rare faunal elements in the vast majority of vertebrate localities. Our knowledge of the occurrence of more than one species in one locality is very likely to be affected by this rarity.
Palaeoenvironment
The ecology as well as locomotion of Chalicotheriidae have repeatedly been discussed (e.g., Cuvier 1823; Kaup 1833; Schaub 1943; Zapfe 1979; Schulz et al. 2007; Schulz and Fahlke 2009; Semprebon et al. 2011). Both subfamilies possibly preferred different habitats, indicated by morphological differences in their teeth and limb proportions. The Chalicotheriinae have shorter tooth crown heights and forelimbs that are much longer than the hindlimbs, an unusual condition also found in extant gorillas. The teeth of Schizotheriinae have higher crowns and their limb proportions are closer to those of other ungulates (Coombs and Cote 2010). Chalicotheriinae are traditionally regarded as browsers, feeding on soft leaves, whereas Schizotheriinae, with their higher tooth crowns, are adjusted to a more abrasive diet. Recent micro- and mesowear analyses of the wear pattern of teeth of chalicotheres revealed that the European representatives of Chalicotherium and Anisodon included probably hard fruits, seeds, or nuts in their diet, whereas Ancylotherium pentelicum mainly fed on leaves, but also consumed bark and twigs (Semprebon et al. 2011). Furthermore, Semprebon et al. (2011) mentioned that regional habitat differences may have been more important, concerning the diet of chalicotheres, than the phylogenetic relationship, since the schizotheriine Metaschizotherium from the Middle Miocene showed meso- and microwear patterns similar to the contemporaneous chalicotheriine Anisodon grande, which also lived in Europe.
The possible ecological differences among chalicotheres might explain the rarity of co-occurrence in one locality and horizon. The fact that during the Turolian age the schizotheriine Ancylotherium pentelicum is frequently found alongside a chalicotheriine in the western part of the Balkan-Iranian zoogeographical province might be interpreted as an ecological indicator. It is known that there were considerable palaeogeographical and palaeoclimatic changes in this area during the Turolian, but the impact of these changes on the habitats is still debated (e.g., Solounias et al. 1999; Kaya et al. 2018; Denk et al. 2018; Fortelius et al. 2019). A global trend of cooling and drying has been documented for the Late Miocene, with its peak in the Messinian stage (Herbert et al. 2016). In the Eastern Mediterranean specifically, an important trend of cooling and aridification has been observed during the latest Tortonian and earliest Messinian (Mertz-Kraus et al. 2008; Kontakiotis et al. 2019). Furthermore, changes in the sea-level of the Paratethys affected the climate in the wider area and most probably shaped the habitats of the Balkan-Iranian zoogeographical province (Butiseacă et al. 2021; Böhme et al. 2021; Palcu et al. 2021), for which a savannah-like palaeoenvironment has been reconstructed (Abel 1922; Bonis et al. 1992; Böhme et al. 2017; Kaya et al. 2018; Fortelius et al. 2019). Despite the fact that some researchers have argued against this hypothesis, based on the faunal and floral composition (Solounias et al. 1999; Denk et al. 2018), the landscape was marked by the existence of open grasslands with the gradual expansion of C4 plants (Cerling et al. 1993; Tzanova et al. 2015).
Overall, it seems that the palaeoenvironment in the Eastern Mediterranean was rather diverse with more local fluctuations of the environmental conditions that controlled the mammalian communities of the Late Miocene (Kostopoulos 2009). An abundance of vertebrate localities in this region exposes a high disparity in the faunal composition regarding the large mammals, even though they are geographically and stratigraphically very restricted (Koufos et al. 2005; Kostopoulos 2009; Koufos 2013). Many studies have investigated this disparity and suggested that climatic changes, migration events, geographical boundaries, or a combination of these factors have affected the composition of the large mammal fauna (e.g., Bonis et al. 1992; Koufos et al. 2005; Kostopoulos 2009; Böhme et al. 2021). This demonstrates the complexity of the faunal assemblages of the Balkan-Iranian zoogeographical province, also known as the “Pikermian Biome”, which was named after the oldest and most famous fossil locality in the region (Solounias et al. 1999).
The chalicotheres that lived during the Turolian in the Balkan-Iranian zoogeographical province also reflect the complexity of habitats. Up to three different species of chalicotheriines have been reported from geographically closely situated fossil localities and probably had rather similar dietary preferences (Bonis et al. 1995; Geraads et al. 2001). Furthermore, our results show that the chalicotheriines were able to coexist with the single schizotheriine species known from the area during this time, Ancylotherium pentelicum (Giaourtsakis and Koufos 2009; Coombs 2013; Kampouridis et al. 2022; Geraads et al. 2007), in at least six different localities in the western part of the Balkan-Iranian zoogeographical province (Fig. 8). This was probably possible because these animals exploited different food sources. Our results also support the notion of a dynamic palaeoenvironment in this region that included both closed and open habitats in close proximity in the Balkan-Iranian zoogeographical province. This supported a variety of ecological niches, which allowed animals even with similar dietary needs to coexist in the same geographical area without having to compete for food resources.
Conclusion
The Upper Miocene locality of Pogana 1 has yielded three specimens that belong to Chalicotheriidae. Two specimens, a previously described distal phalanx and the herein described proximal phalanx, belong to the schizotheriine Ancylotherium pentelicum, the most typical representative of this family during the Upper Miocene of the Balkan-Iranian zoogeographical province. The third specimen, a partial calcaneum, is herein assigned to Chalicotheriinae indet. The chalicotheriine calcaneum was found in the same fossil site and horizon as the Ancylotherium pentelicum material. Thus, Pogana is one of the few certain cases where the co-occurrence of Schizotheriinae and Chalicotheriinae in a single locality and horizon is confirmed. In total, six out of eight such cases are found in the Balkan-Iranian zoogeographical province during the Late Miocene, all of Turolian age. Recent studies have suggested that representatives of the two subfamilies may have had rather similar dietary preferences. Therefore, their frequent co-occurrence in the Turolian of the Balkan-Iranian zoogeographical province, in contrast to their separate occurrences at almost any other time period supports the notion of a rich mosaic environment that included both closed forest and more open environments. It also indicates that the co-occurrence of Schizotheriinae and Chalicotheriinae might have been controlled by provincial changes in the environment.
Data availability
All data generated during this study are included in this published article.
References
Abel O (1922) Lebensbilder aus der Tierwelt. Verlag von Gustav Fischer, Jena
Anquetin J, Antoine P-O, Tassy P (2007) Middle Miocene Chalicotheriinae (Mammalia, Perissodactyla) from France, with a discussion on chalicotheriine phylogeny. Zool J Linn Soc 151:577–608. https://doi.org/10.1111/j.1096-3642.2007.00327.x
Antoine P-O, Métais G, Orliac M, Crochet J-Y, Flynn LJ, Marivaux L, Rajpar AR, Roohi G, Welcomme J-L (2013) Mammalian Neogene biostratigraphy of the Sulaiman Province, Pakistan. In: Wang X, Flynn LJ, Fortelius M (eds) Neogene Mammals of Asia. Columbia University Press, New York, pp 400–422
Bai B (2008) A review on Chinese Eocene chalicotheres Eomoropus and Grangeria. In: Dong W (ed) Proceedings of the Eleventh Annual Meeting of the Chinese Society of Vertebrate Paleontology. China Ocean Press, Beijing, pp 19–30
Bai B, Wang Y, Meng J (2010) New craniodental materials of Litolophus gobiensis (Perissodactyla, “Eomoropidae”) from Inner Mongolia, China, and phylogenetic analyses of Eocene Chalicotheres. Am Mus Novit 3688:1–27. https://doi.org/10.1206/678.1
Boev ZN (2017) Fossil records of rhinoceroses (Rhinocerotoidea Gray, 1821), chalicotheres (Chalicotherioidea Gill, 1872) and brontotheres (Brontotherioidea (Marsh, 1873) (Peryssodactyla Owen, 1848 - Mammalia Linnaeus, 1758) in Bulgaria. Bull Nat Hist Mus Plovdiv 2:1–7
Böhme M, Spassov N, Ebner M, Geraads D, Hristova L, Kirscher U, Kötter S, Linnemann U, Prieto J, Roussiakis S, Theodorou G, Uhlig G, Winklhofer M (2017) Messinian age and savannah environment of the possible hominin Graecopithecus from Europe. PLoS ONE 12:e0177347. https://doi.org/10.1371/journal.pone.0177347
Böhme M, Van Baak CGC, Prieto J, Winklhofer M, Spassov N (2018) Late Miocene stratigraphy, palaeoclimate and evolution of the Sandanski Basin (Bulgaria) and the chronology of the Pikermian faunal changes. Global Planet Change 170:1–19. https://doi.org/10.1016/j.gloplacha.2018.07.019
Böhme M, Spassov N, Majidifard MR, Gärtner A, Kirscher U, Marks M, Dietzel C, Uhlig G, El Atfy H, Begun DR, Winklhofer M (2021) Neogene hyperaridity in Arabia drove the directions of mammalian dispersal between Africa and Eurasia. Commun Earth Environ 2:85. https://doi.org/10.1038/s43247-021-00158-y
Bonis L de (1999) Palaeoenvironments of Late Miocene primate localities in Macedonia, Greece. In: Agusti J, Rook L, Andrews P (eds) The Evolution of Neogene Terrestrial Ecosystems in Europe. Cambridge University Press, Cambridge, UK, pp 425–435
Bonis L de, de Brunet M, Heintz E, Sen S (1992) La province Greco-irano-afghane et la répartition des faunes mammaliennes au Miocène supérieur. Paleontol Evol 24–25:103–112
Bonis L de, Bouvrain G, Koufos G, Tassy P (1995) Un crane de chalicothere (Mammalia, Perissodactyla) du Miocene superieur de Macedoine (Grece): remarques sur la phylogenie des Chalicotheriinae. Palaeovertebrata 24:135–176
Butiseacă GA, Vasiliev I, van der Meer MTJ, Krijgsman W, Palcu DV, Feurdean A, Niedermeyer EM, Mulch A (2021) Severe Late Miocene droughts affected western Eurasia. Global Planet Change 206:103644. https://doi.org/10.1016/j.gloplacha.2021.103644
Butler PM (1965) Fossil mammals of Africa No. 18: East African Miocene and Pleistocene chalicotheres. Bull Brit Mus Geol 10:163–237
Cerling TE, Wang Y, Quade J (1993) Expansion of C4 ecosystems as an indicator of global ecological change in the Late Miocene. Nature 361:344–345. https://doi.org/10.1038/361344a0
Chen S (2008) A review on Chinese Neogene chalicotheres. In: Dong W (ed) Proceedings of the Eleventh Annual Meeting of the Chinese Society of Vertebrate Paleontology. China Ocean Press, Beijing, pp 31–41
Chen S, Deng T, Pang L, He W, Chen S (2012) A juvenile skull of Ancylotherium (Mammalia, Perissodactyla, Chalicotheriidae) from the Pliocene of China. Geobios 45:527–534. https://doi.org/10.1016/j.geobios.2012.06.002
Codrea VA, Bordeianu M, Solomon AA (2019) The first record of the chalicothere Ancylotherium pentelicum in Romania. Muzeul Olteniei Craiova Oltenia Studii şi comunicări Ştiinţele Naturii 35:37–44
Colbert EH (1934) Chalicotheres from Mongolia and China in the American Museum. Bull Am Mus Nat Hist 67:353–387
Colbert EH (1935) Distribution and phylogenetic studies on Indian mammals. III A classification of the Chalicotheroidea. Am Mus Novit 798:1–16
Coombs MC (1978a) Reevaluation of the early Miocene North American Moropus (Perissodactyla, Chalicotheriidae, Schizotheriinae). Bull Carn Mus Nat Hist 4:1–62
Coombs MC (1978b) Additional Schizotherium material from China, and a review of Schizotherium dentitions (Perissodactyla, Chalicotheriidae). Am Mus Novit 2647:1–18
Coombs MC (1979) Tylocephalonyx, a new genus of North American dome-skulled chalicotheres (Mammalia, Perissodactyla). Bull Am Mus Nat Hist 164:1–68
Coombs MC (1983) Large mammalian clawed herbivores: A comparative study. Trans Am Phil Soc 73:1. https://doi.org/10.2307/3137420
Coombs MC (1989) Interrelationship and diversity in the Chalicotheriidae. In: The Evolution of Perissodactyls. Oxford University Press, New York
Coombs MC (2009) The chalicothere Metaschizotherium bavaricum (Perissodactyla, Chalicotheriidae, Schizotheriinae) from the Miocene (MN5) Lagerstätte of Sandelzhausen (Germany): description, comparison, and paleoecological significance. Paläontol Z 83:85–129. https://doi.org/10.1007/s12542-009-0004-x
Coombs MC (2013) A juvenile mandible with deciduous teeth of Ancylotherium pentelicum (Perissodactyla, Chalicotheriidae, Schizotheriinae), collected by Barnum Brown from the Late Miocene of Samos (Greece). J Vertebr Paleontol 33:233–238. https://doi.org/10.1080/02724634.2012.710281
Coombs MC, Cote SM (2010) Chalicotheriidae. In: Werdelin L, Sanders WJ (eds) Cenozoic Mammals of Africa. University of California Press, pp 659–668
Coombs MC, Göhlich UB (2019) Anisodon (Perissodactyla, Chalicotheriinae) from the Middle Miocene locality Gračanica (Bugojno Basin, Gornji Vakuf, Bosnia and Herzegovina). Palaeobio Palaeoenv. https://doi.org/10.1007/s12549-018-0357-9
Coombs MC, Rothschild BM (1999) Phalangeal fusion in schizotheriine chalicotheres (Mammalia, Perissodactyla). J Paleontol 73:682–690. https://doi.org/10.1017/S0022336000032509
Cuvier G (1823) Sur une phalange ongueale fossile qui annonce a elle seule un Edente inconnu, probablement du genre des pangolins, et de taille giantesque. Recherches sur les Ossemens fossils 5:193–195
Dehm R (1980) Über ein neues Hyotherium (Suidae, Schweine-Verwandte) aus der Oberen Süßwassermolasse Südbayerns. Ann Naturhist Mus Wien 83:49–57
Deng T, Hou S, Shi Q, Chen S, He W, Chen S (2011) Terrestrial Mio-Pliocene Boundary in the Linxia Basin, Gansu, China. Acta Geol Sin 85:452–464. https://doi.org/10.1111/j.1755-6724.2011.00413.x
Denk T, Zohner CM, Grimm GW, Renner SS (2018) Plant fossils reveal major biomes occupied by the Late Miocene Old-World Pikermian fauna. Nat Ecol Evol 2:1864–1870. https://doi.org/10.1038/s41559-018-0695-z
Depéret C (1892) Le faune de mammifères miocènes de La Grive-Saint-Alban (Isère) et quelques autres localités du bassin du Rhône. Arch Mus Hist Nat Lyon 5:1–93
Fahlke JM, Coombs MC (2009) Dentition and first postcranial description of Metaschizotherium fraasi Koenigswald, 1932 (Perissodactyla: Chalicotheriidae) and its occurrence on a karstic plateau – new insights into schizotheriine morphology, relationships, and ecology. PalA 290:65–129. https://doi.org/10.1127/pala/290/2009/65
Fahlke JM, Coombs MC, Semprebon GM (2013) Anisodon sp. (Mammalia, Perissodactyla, Chalicotheriidae) from the Turolian of Dorn-Dürkheim 1 (Rheinhessen, Germany): morphology, phylogeny, and palaeoecology of the latest chalicothere in Central Europe. Palaeobio Palaeoenv 93:151–170. https://doi.org/10.1007/s12549-013-0119-7
Fortelius M, Eronen JT, Kaya F, Tang H, Raia P, Puolamäki K (2014) Evolution of Neogene mammals in Eurasia: environmental forcing and biotic Interactions. Annu Rev Earth Planet Sci 42:579–604. https://doi.org/10.1146/annurev-earth-050212-124030
Fortelius M, Bibi F, Tang H, Žliobaitė I, Eronen JT, Kaya F (2019) The nature of the Old World savannah palaeobiome. Nat Ecol Evol 3:504–504. https://doi.org/10.1038/s41559-019-0857-7
Garevski R (1974) Beitrag zur Kenntins der Pikermifauna Mazedoniens. Fossilreste der Chalicotheriiden. Fragm Balc 9:201–209
Garevski R, Zapfe H (1983) Weitere Chalicotheriiden-Funde aus der Pikermi-Fauna von Titov Veles (Mazedonien, Jugoslawien). Acta Mus Macedonici Scient Nat 17:1–20
Gaudry A (1862–1867) Animaux fossiles et géologie de l’Attique. F. Savy, Paris
Gaudry A, Lartet E (1856) Sur les résultats des recherches paléontologiques entreprises dans l’Attique sous les auspices de l’Académie. C R Séances Acad Sci 43:271–274
Geraads D, Spassov N, Kovachev D (2001) New Chalicotheriidae (Perissodactyla, Mammalia) from the Late Miocene of Bulgaria. J Vertebr Paleontol 21:596–606. https://doi.org/10.1671/0272-4634(2001)021[0596:NCPMFT]2.0.CO;2
Geraads D, Spassov N, Kovachev D (2006) The Bulgarian Chalicotheriidae (Mammalia): an update. Rev Paleobio Genève 25:429–437
Geraads D, Tsoukala E, Spassov N (2007) A skull fo Ancylotherium (Chalicotheriidae, Mammalia) from the late Miocene of Thermopigi (Serres, N. Greece) and the relationships of the genus. Journal of Vertebrate Paleontology 27(2):461–466
Giaourtsakis IX, Koufos GD (2009) The Late Miocene mammal faunas of the Mytilinii Basin, Samos Island, Greece: New collection. 10. Chalicotheriidae. Beitr Paläontol 31:189–205
Gill T (1872) Arrangements of the families of mammals with analytical tables. Smithson Misc Coll 11:1–98
Handa N, Nakatsukasa M, Kunimatsu Y, Tsubamoto T, Nakaya H (2021) The Chalicotheriidae (Mammalia, Perissodactyla) from the Upper Miocene Nakali Formation, Kenya. Hist Biol 33:3522–3529. https://doi.org/10.1080/08912963.2021.1876042
Heissig K (1999) Family Chalicotheriidae. In: The Miocene Land Mammals of Europe. Pfeil, Munich, pp 189–192
Herbert TD, Lawrence KT, Tzanova A, Peterson LC, Caballero-Gill R, Kelly CS (2016) Late Miocene global cooling and the rise of modern ecosystems. Nature Geosci 9:843–847. https://doi.org/10.1038/ngeo2813
Holland W, Peterson O (1914) The osteology of the Chalicotheroidea with special reference to a mounted skeleton of Moropus elatus Marsh, now installed in the Carnegie Museum. Mem Carn Mus 3:189–406
Hristova L, Kovachev D, Spassov N (2003) Hipparion brachypus Hensel, 1862 from Hadjidimovo, southwestern Bulgaria (Late Miocene). CR Acad Bulg Sci 56:77–84
Ionesi L (1994) Geology of the platform units and of North Dobrogea orogen. Editura Tehnica, București
Jeanrenaud P (1971) Geologia Moldovei Centrale dintre Siret şi Prut. PhD Dissertation, „Al.I. Cuza”
Kampouridis P, Roussiakis SJ, Giaourtsakis IX, Kargopoulos N, Svorligkou G, Theodorou GE (2022) Ancylotherium pentelicum (Mammalia, Chalicotheriidae) from the Late Miocene of Kerassia (Greece) and remarks on its intraspecific variability. Palaeobio Palaeoenv 102:193–203. https://doi.org/10.1007/s12549-021-00497-w
Kaup J (1833) Der Krallen-Phalanx von Eppelsheim, nach welchem Herr von Cuvier seinen Riesen-Pangolin, Manis gigantea, aufstellte, gehört zu Dinotherium. Neues Jahrb Mineral Geol Palaontol 1833:172–176
Kaya F, Bibi F, Žliobaitė I, Eronen JT, Hui T, Fortelius M (2018) The rise and fall of the Old World savannah fauna and the origins of the African savannah biome. Nat Ecol Evol 2:241–246. https://doi.org/10.1038/s41559-017-0414-1
Koenigswald GHR von (1932) Metaschizotherium fraasi n. g. n. sp., ein neuer Chalicotheriidae aus dem Obermiocän von Steinheinm a. Albuch. Beitr Naturgesch Vorz Supp Bd 8:1–24
Kontakiotis G, Besiou E, Antonarakou A, Zarkogiannis SD, Kostis A, Mortyn PG, Moissette P, Cornée J-J, Schulbert C, Drinia H, Anastasakis G, Karakitsios V (2019) Decoding sea surface and paleoclimate conditions in the eastern Mediterranean over the Tortonian-Messinian Transition. Palaeogeo Palaeoclim Palaeoeco 534:109312. https://doi.org/10.1016/j.palaeo.2019.109312
Kostopoulos DS (2009) The Pikermian Event: Temporal and spatial resolution of the Turolian large mammal fauna in SE Europe. Palaeogeo Palaeoclim Palaeoeco 274:82–95. https://doi.org/10.1016/j.palaeo.2008.12.020
Koufos GD (2012) New material of Chalicotheriidae (Perissodactyla, Mammalia) from the Late Miocene of Axios Valley, Macedonia (Greece) with the description of a new species. Ann Paleo 98:203–224. https://doi.org/10.1016/j.annpal.2012.06.002
Koufos GD (2013) Neogene mammal biostratigraphy and chronology of Greece. In: Wang X, Flynn JJ, Fortelius M (eds) Fossil Mammals of Asia. Neogene Biostratigraphy and Chronology. Columbia University Press, New York, pp 595–621
Koufos GD, Kostopoulos DS, Vlachou TD (2005) Neogene/Quaternary mammalian migrations in Eastern Mediterranean. Belg J Zool 135:181–190
Li X-C, Deng K (2003) Early Pleistocene chalicothere fossils from Huangjiawan, Zhen’an, Shaanxi, China. Verteb PalAsia 41:332–336
Li Z, Mörs T, Zhang Y, et al (2022) New material of schizotheriine chalicothere (Perissodactyla, Chalicotheriidae) from the Xianshuihe Formation (Early Miocene) of Lanzhou Basin, Northwest China. J Mammal Evol. https://doi.org/10.1007/s10914-022-09619-3
Linnaeus C (1758) Systema Naturae. Laurentii Salvii, Stockholm
Liu Y, Zhang Z (2012) New materials of Chalicotherium brevirostris (Perissodactyla, Chalicotheriidae) from the Tunggur Formation, Inner Mongolia. Geobios 45:369–376. https://doi.org/10.1016/j.geobios.2011.10.011
Mein P, Ginsburg L (2002) Sur l’âge relatif des différents dépôts karstiques miocènes de La Grive-Saint-Alban (Isère). Cah Sci Mus Hist Nat Lyon 5:7–47. https://doi.org/10.3406/mhnly.2002.1328
Mertz-Kraus R, Brachert TC, Reuter M (2008) Tarbellastraea (Scleractinia): A new stable isotope archive for Late Miocene paleoenvironments in the Mediterranean. Palaeogeo Palaeoclim Palaeoeco 257:294–307. https://doi.org/10.1016/j.palaeo.2007.10.023
Morales J, Nieto M, Köhler M, Moyà-Solà S (1999) Large mammals from the Vallesian of Spain. In: Agusti J, Rook L, Andrews P (eds) Hominoid Evolution and Climate Change in Europe: The Evolution of the Neogene Terrestrial Ecosystems in Europe. Cambridge University Press, Cambridge, UK, pp 113–126
Owen R (1848) Description of teeth and portions of jaws of two extinct anthracotherioid quadrupeds (Hyopotamus vectianus and Hyop. bovinus) discovered by the Marchioness of Hastings in the Eocene deposits on the N.W. coast of the Isle of Wight; with an attempt to develop Cuvier’s idea of the classification of pachyderms by the number of their toes. Quart J Geol Soc London 4:103–141
Palcu DV, Patina IS, Șandric I, Lazarev S, Vasiliev I, Stoica M, Krijgsman W (2021) Late Miocene megalake regressions in Eurasia. Sci Rep 11:11471. https://doi.org/10.1038/s41598-021-91001-z
Pickford M (2020) Description catalogue of Chalicotheriidae (Mammalia, Perissodactyla) from the Early Miocene of Napak, Uganda. Geol-Pal Uganda 15:1–36
Qiu Z, Wang B, Xie J (1998) Mid-Tertiary chalicothere (Perissodactyla) fossils from Lanzhou, Gansu, China. Verteb PalAsia 36:297–318
Roussiakis S, Athanassiou A, Michailidis D, Mitsopoulou V, Solomos C, Theodorou GE (2014) Remarks on new proboscidean remains from the classical Late Miocene locality of Pikermi and their associated fauna. Scientific Annals, School of Geology, Aristotle University of Thessaloniki, Greece VIth International Conference on Mammoths and their Relatives, Grevena - Siatista 102:171–172
Roussiakis S, Filis P, Sklavounou S, Giaourtsakis IX, Kargopoulos N, Theodorou GE (2019) Pikermi: a classical European fossil mammal geotope on the spotlight. Eur Geol 48:28–32
Roussiakis S, Theodorou GE (2001) Ancylotherium pentelicum (Gaudry and Lartet, 1856) (Perissodactyla, Mammalia) from the classic locality of Pikermi (Attica, Greece), stored in the Palaeontological and Geological Museum of Athens. Geobios 34:563–584
Săndulescu M (1984) Geotectonica României. Editura Tehnica, București
Saraç G, Sen S (2005) Chalicotheriidae (Mammalia, Perissodactyla) from the Late Miocene of Akkasdagi, Turkey. Geodiversitas 27:591–600
Schaefer H, Zapfe H (1971) Chalicotherium grande Blainv. und Chalicotherium goldfussi Kaup. Odontologische und osteologische Unterschiede. Verhandlungen der Naturforschenden Gesellschaft in Basel 81:157–199
Schaub S (1943) Vorderestremität von Ancylotherium pentelicum Gaudry und Lartet. Schweiz Palaeontol Abh 64:1–36
Schlosser M (1921) Die Hipparionenfauna von Veles in Mazedonien. Abh Bayer Akad Wiss 24:1–55
Schulz E, Fahlke JM (2009) The diet of Metaschizotherium bavaricum (Chalicotheriidae, Mammalia) from the MN 5 of Sandelzhausen (Germany) implied by the mesowear method. Paläontol Z 83:175–181. https://doi.org/10.1007/s12542-009-0007-7
Schulz E, Fahlke JM, Merceron G, Kaiser TM (2007) Feeding ecology of the Chalicotheriidae (Mammalia, Perissodactyla, Ancylopoda). Results from dental micro- and mesowear analyses. Verh Naturwiss Ver Hamburg 43:5–31
Semprebon GM, Sise PJ, Coombs MC (2011) Potential bark and fruit browsing as revealed by stereomicrowear analysis of the peculiar clawed herbivores known as chalicotheres (Perissodactyla, Chalicotherioidea). J Mammal Evol 18:33–55. https://doi.org/10.1007/s10914-010-9149-3
Solounias N, Plavcan JM, Quade J, Witmer L (1999) The paleoecology of the Pikermian Biome and the savanna myth. In: Agustí J, Rook L, Andrews P (eds) Hominoid Evolution and Climatic Change in Europe, 1st edn. Cambridge University Press, pp 436–453
Spassov N, Tzankov T, Geraads D (2006) Late Neogene stratigraphy, biochronology, faunal diversity and environments of South-West Bulgaria (Struma River Valley). Geodiversitas 28:477–498
Spassov N, Geraads D, Hristova L, Markov GN, Garevska B, Garevski R (2018) The late Miocene mammal faunas of the Republic of Macedonia (FYROM). PalA 311:1–85. https://doi.org/10.1127/pala/2018/0073
Spassov N, Geraads D, Markov G (2019) The late Miocene mammal fauna from Gorna Sushitsa, southwestern Bulgaria, and the early/middle Turolian transition. Neues Jahrb Geol Palaontol Abh 291:317–350. https://doi.org/10.1127/njgpa/2019/0804
Symeonidis N, Bachmayer F, Zapfe H (1973) Ausgrabungen in Pikermi bei Athen, Griechenland. Ann Naturh Mus Wien 77:125–132
Symeonidis NK (1973) Chalicotherium goldfussi Kaup (Perissodactyla, Mammalia) aus dem Altpliozän von Pikermi (Griechenland). Ann Geo Pays Helléniques 25:301–307
Theodorou GE, Roussiakis SJ, Athanassiou A, Filippidi A (2010) Mammalian remains from a new site near the classical locality of Pikermi (Attica, Greece). Sci Ann School Geol 99:109–119
Tsoukala E (2022) The fossil record of chalicotheres (Mammalia: Perissodactyla: Chalicotheriidae) in Greece. In: Vlachos E (ed) Fossil Vertebrates of Greece Vol. 2. Springer International Publishing, Cham, pp 501–517
Tzanova A, Herbert TD, Peterson L (2015) Cooling Mediterranean Sea surface temperatures during the Late Miocene provide a climate context for evolutionary transitions in Africa and Eurasia. Earth Planet Sci Lett 419:71–80. https://doi.org/10.1016/j.epsl.2015.03.016
Ursachi L (2016) Asociații de vertebrate continentale miocene din zona centrala a Podisului Barladului. PhD Dissertation, University Alexandru Ioan Cuza
Wagner A (1848) Urweltliche Säugetier-Überreste aus Griechenland. Abh Bayer Akad Wiss 5:335–378
Welcomme J-L, Benammi M, Crochet J-Y, et al (2001) Himalayan Forelands: palaeontological evidence for Oligocene detrital deposits in the Bugti Hills (Balochistan, Pakistan). Geol Mag 138:397–405. https://doi.org/10.1017/S0016756801005428
Zapfe H (1979) Chalicotherium grande (Blainville) aus der miozänen Spaltenfüllung von Neudorf an der March (Devinska Nova Ves), Tschechoslowakei. Neue Denkschr Naturh Mus Wien 2:1–282
Acknowledgements
We would like to thank P.-O. Antoine (Montpellier, France), S. Chen (Beijing, China), M. C. Coombs (Massachusetts, USA), A. Matzke (Tübingen, Germany), S. Roussiakis (Athens, Greece) and N. Spassov (Sofia, Bulgaria) for fruitful discussions and for providing important information and literature about the Eurasian chalicotheres. We thank U. Göhlich (Vienna, Austria) for providing acces to the chalicothere material housed in the collections of the NHMW in Austria. We thank the reviewers M. Coombs (Massachusetts, USA) and N. Handa (Nara, Japan) and the Associate Editor P. Coster (Kansas, USA) for helpful comments and suggestions that significantly improved the quality of the manuscript. Many thanks go to Mocanu Dumitru, Fernando Perniciano, Simon Salca, Adrian Andrei, and all excavators in the field who made this work possible. We are also grateful to all mayors from Pogana from 2012 to present (Gîfu Maricel, Spiridon Cristi and Vezeteu Ioan).
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported for one of us (R.B.G.) by a grant of the Ministry of Research, Innovation and Digitization, CNCS—UEFISCDI, project number PN-III-P1-1.1-TE-2021–0664, within PNCDI III. This research received support from the SYNTHESYS + project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the H2020 Integrating Activities Programme, Project number 823827 (AT-TAF-TA3-9 and AT-TAF-TA4-29 to P.K.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. B.G.R. and L.U. conducted fieldwork and collected the specimens described, P.K. and B.G.R. wrote the main manuscript text, B.G.R. and L.U. prepared Fig. 1, P.K. prepared Figs. 2–8. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kampouridis, P., Rățoi, B.G. & Ursachi, L. New evidence for the unique coexistence of two subfamilies of clawed perissodactyls (Mammalia, Chalicotheriidae) in the Upper Miocene of Romania and the Eastern Mediterranean. J Mammal Evol 30, 641–656 (2023). https://doi.org/10.1007/s10914-023-09657-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-023-09657-5