Abstract
The Atlantic wolffish (Anarhichas lupus) was assessed as a species of Special Concern by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) under the Canadian Species-At-Risk Act (SARA) in 2001, and by the National Marine Fisheries Service in USA in 2004. Monitoring of marine Species-At-Risk would rely ideally on non-destructive methods. However, most monitoring of marine fish at-risk rely on trawl surveys that are potentially destructive of the environment. Inferring a species presence using environmental DNA (eDNA) detections offers an attractive alternative for Species-At-Risk monitoring, because it is non-destructive, specific, and sensitive. We developed and optimized a real-time quantitative PCR probe-based (qPCR) detection protocol that targets the eDNA of Atlantic wolffish, A. lupus. The qPCR protocol was validated in silico, in vitro, and in situ. Species-specificity was assessed in vitro by testing against the two other species of Anarhichas present in the northwest Atlantic. We did not observe DNA amplification for either of these two species. The assay was highly sensitive, with a limit of detection (95% confidence level) of 1.5 DNA copies per qPCR reaction. In situ tests showed that A. lupus eDNA is detected from expected depth strata in areas of known wolffish abundance. This study provides a proof-of-concept experiment that offers a robust, targeted, and non destructive protocol for detection eDNA of the Atlantic wolffish.
Similar content being viewed by others
Introduction
Environmental DNA (eDNA) is “DNA collected from an environmental sample without any attempt to isolate the organism(s) from which the DNA derives” (Darling 2019). Detection methods of eDNA are used to infer the presence of a species with a non-destructive approach in terrestrial and aquatic environments (Moran et al. 2019; Antognazza et al. 2019; Leempoel et al. 2020). Furthermore, a targeted detection method of eDNA by quantitative polymerase chain reaction (qPCR) can be more sensitive than traditional methods for low-abundance species (Beng and Corlett 2020; Keller et al. 2022). Such non negligeable advantage most likely underlies the gain in popularity of eDNA detections from water samples during the last decade for the monitoring of rare species from freshwater (Thomsen et al. 2012; Wilcox et al. 2013; Rees et al. 2014; Davy et al. 2015; Veldhoen et al. 2016; Roux et al. 2020) and marine environments (Gargan et al. 2017; Weltz et al. 2017; Stoeckle et al. 2018; Wood et al. 2019; Hansen et al. 2020; Chevrinais et al. 2023).
Three species of wolffish, in the genus Anarhichas, occur in the northwest Atlantic Ocean (Kulka et al. 2007). These are A. denticulatus (northern or broadhead wolffish), A. minor (spotted wolffish), and A. lupus (Atlantic or striped wolffish). The three species inhabit shelves from the southern Grand Banks to the Davis Strait (Kulka and DeBlois 1996). A. lupus differed from the two other species by being densely concentrated on the shallow part of the southern Grand Banks (Kulka and DeBlois 1996). This species is also present along the Labrador Shelf, in deep parts of the Gulf of St. Lawrence, on the Scotian Shelf, in the Bay of Fundy (McRuer et al. 2000), along the coast of Maine and on Georges Bank (Nelson and Ross 1992). Higher densities of A. lupus are observed between 150 and 350 m at temperatures between 1.5 and 4 °C (Kulka et al. 2007), and lower densities are also surveyed in shallow waters (< 22 m) (Novaczek et al. 2017). Atlantic wolffish are largely sedentary, remaining in a 4–8 km area during their adult stage (Templeman 1984; Simpson et al. 2014). Adults may undergo seasonal migration from shallow water (< 120 m) in spring to deeper water in autumn (Nelson and Ross 1992). Those fish inhabit caves in boulder bedrocks (Novaczek et al. 2017). In the northwest Atlantic, juveniles of the Atlantic wolffish occur in deeper offshore waters (> 30 m) than adults and adults seem to operate a migration to shallow waters (< 30 m) at sexual maturity to begin to spawn in late August (Keats et al. 1986).
A decline in the density of A. lupus was recorded between 1980 and 2001 in Canadian waters based on trawl surveys (Kulka et al. 2007). In 2001, this species was assessed by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) as of “Special Concern”, i.e., a species that might become Threatened or Endangered if trends continue. It is the subject of a management plan formulated in 2007 to increase its densities and distribution, and thus achieve long-term viability of this species. Anarhichas lupus is still vulnerable as bycatch in 20 directed demersal fisheries (Kulka et al. 2007; Bluemel et al. 2022). Trawl survey monitoring is also the primary source of information for monitoring A. lupus. However, some A. lupus habitats such as boulder bedrocks are unsuitable for trawling and no occurrence information is available in these areas. Environmental DNA monitoring would be a complementary non-destructive method providing occurrence information across the species distribution and limiting mortality due to trawling.
In this study, we developed and optimized a qPCR assay to detect DNA from Atlantic wolffish with in silico, in vitro and in situ validation steps. Our aim was to provide a new molecular and non-destructive tool for the detection (i.e., presence, absence) of this species. A probe-based qPCR assay was developed following the MIQE (Minimum Information for Publication of Quantitative Real-Time PCR experiments, Bustin et al. 2009), the critical considerations for the application of eDNA methods to detect aquatic species (Goldberg et al. 2016), the Fisheries and Oceans Canada (DFO) minimum requirements in eDNA studies (Abbott et al. 2021), and the Canadian Standards Association eDNA reporting requirements and terminology (Gagne et al. 2021). We report a detailed protocol for the qPCR assay, an evaluation of performance characteristics, and an initial field test of the detection protocol as an eDNA biomonitoring tool for a Species-At-Risk.
Materials and methods
In silico design
A partial sequence of the mitochondrial cytochrome B (CYTB) gene was targeted because interspecific variation for the Anarhichas genus was previously documented (Johnstone et al. 2007; Lait and Carr 2018). Sequences from west Atlantic specimens were retrieved from NCBI (National Center for Biotechnology Information, nih.gov; Table S1). They were aligned with Geneious Prime 2020.0.4 (https://www.geneious.com) with the ClustalW alignment to identify conserved and variable regions in A. lupus and closely related species. Specific primers and TaqMan™ Minor Groove Binding (MGB) probe were designed manually to amplify a region of 92 base pairs (bp). Mitochondrial DNA fragments under 200 bp were targeted to maximize the likelihood of detecting short fragments of eDNA (Jo et al. 2022). Assay specificity was tested in silico with Geneious Prime 2020.0.4 with sequences from closely related species and using Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (Table S1, Table 1). The qPCR amplification parameters were optimized using OligoEvaluator (http://www.oligoevaluator.com) and Tm Calculator (http://tmcalculator.neb.com). The qPCR assay was then optimized for an annealing temperature of 60 °C.
In vitro specificity
In vitro specificity of the qPCR assay was tested with DNA extracts from congeneric species of A. lupus, A. denticulatus, and A. minor (Table S2). Genomic DNA was extracted from each specimen using the DNeasy® Blood and Tissue kit (QIAGEN, Hilden, Germany) following the manufacturer’s protocol and was stored at -20 °C.
Nonspecific amplification and primer dimer amplification were assessed by specificity testing with TaqMan® probe qPCR. The volumes and concentrations of qPCR assay solution were 12.5 µL of TaqMan™ Gene expression master mix (Thermo Fisher Scientific™, Waltham, USA), 1.2 µL of forward and reverse primers (10 µM), 0.5 µL of the TaqMan™ MGB probe (10 µM, Applied Biosystems®, Foster City, USA), 3 µL of DNA template, 1 µL of 1% Bovine Serum Albumin (Sigma-Aldrich, Saint-Louis, MO, USA), and 5.6 µL of nuclease-free water (Sigma-Aldrich, Saint-Louis, MO, USA) to yield a volume of 25 µL per reaction. Cycling parameters were 2 min. at 50 °C, 10 min. at 95 °C followed by 40 cycles of 30 s. denaturation step at 95 °C, 30 s. annealing step at 60 °C, and 30 s. extension step at 72 °C. All qPCR reactions were conducted on the AriaMX qPCR system (Agilent Technologies, Santa Clara, CA, USA). Fluorescence was measured at the end of each elongation step.
In vitro optimization
Optimal primer and probe concentrations were defined as the conditions generating the lowest quantification cycle (Cq value) and were assessed using plasmid DNA (pDNA, Genewiz, South Plainfield, NJ, USA) containing the target CYTB sequence. Primer concentrations were first optimized asymmetrically, testing forward and reverse primer final reaction concentrations with combinations of 200, 400, 600 and 800 nM and a fixed probe concentration of 200 nM (Fig. S1A). Then, probe concentrations of 100, 150, 200, and 250 nM were tested with the best primer concentrations combination (Fig. S1B). A last qPCR test was performed to assess the impact of low template DNA concentrations (20 to 2 DNA copies per qPCR reaction) with the optimal primers and probe concentrations (Fig. S1C).
The optimized volumes and concentrations of qPCR assay solutions were 10 µL of TaqPath™ ProAmp™ 2x master mix (Thermo Fisher Scientific™, Waltham, USA), 1.6 µL of forward and 1.2 µL of reverse primer (10 µM), 0.5 µL of the TaqMan™ MGB probe (10 µM, Applied Biosystems®, Foster City, USA), 3 µL of DNA template, 0.8 µL of 1% Bovine Serum Albumin (Sigma-Aldrich, Saint-Louis, MO, USA), and 2.9 µL of nuclease-free water (Sigma-Aldrich, Saint-Louis, MO, USA) to yield a volume of 20 µL per reaction. Cycling parameters were 10 min. at 95 °C followed by 45 cycles of 15 s. denaturation step at 95 °C, and 1 min. annealing step at 60 °C with the same apparatus as in the previous section.
In vitro sensitivity
Serial dilutions of pDNA were used to determine the in vitro sensitivity of the qPCR assay with optimal qPCR conditions from Sect. 2.3. Serial dilutions from 20,000 to 0.25 DNA copies per reaction were tested in six replicates by qPCR to determine the assay efficiency using the equation E = -1 + 10[-1/slope] and to calculate the theoretical limit of detection (LOD). The LOD95% was defined as the lowest standard concentration at which 95% of the replicates had pDNA detected. The LOD95% is determined following the method developed by Klymus et al. (2019).
In situ validation
Samples were processed following a qPCR detection workflow with procedures to limit contamination (Chevrinais et al. 2023, Fig. 1, Appendix 1). Briefly, all samples were processed in an eDNA specific ultraclean laboratory, located at the Maurice Lamontagne Institute (MLI, Fisheries and Oceans Canada, Quebec). All laboratory users were trained to work in clean conditions according to a standard operating procedure.
Sampling and filtration
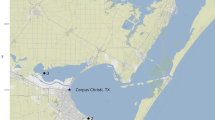
The qPCR assay was validated in situ with water samples from the 2020 DFO annual ecosystem survey in the Estuary and Gulf St. Lawrence. Samples were collected from six stations of the Gulf of St. Lawrence (Fig. 1). Four stations (1–4, Fig. 1) and two stations (5, 6, Fig. 1) were in areas of high and low densities of A. lupus, respectively, according to trawl captures with a four-sided Campelen 1800 shrimp trawl equipped with a Rockhopper footgear (McCallum and Walsh 2002; Bourdages et al. 2021). At each station, two 2 L samples were collected from two distinct Niskin bottles, i.e. one at 15 m from the surface and the other at one m from the bottom (Fig. 1, Nsamples = 12). Samples were frozen at – 20 °C until filtration at MLI. Along with field samples, a field negative control was collected each sampling day. It consisted in Milli-Q® water (MilliporeSigma, Darmstadt, Germany) transferred between two 2 L bottles in the vessel sampling room.
After overnight thawing, samples and field negative controls were filtered with 1.2 μm glass fibre filters using a vacuum pump filtering system in the ultraclean laboratory (Chevrinais et al. 2023, Appendix 4). A filtration negative control using Milli-Q® water was included on each filtration day. Filters were preserved at -20 °C until extraction.
Sampling stations in the Gulf of St. Lawrence (Canada) and qPCR replicate results for Anarhichas lupus qPCR assay. Results from the three qPCR replicates per depth are presented (15 m: upper squares; bottom: lower squares). Black squares indicate positive qPCR replicates, while empty squares indicate negative qPCR replicates (WGS 84/Pseudo-Mercator EPSG:3857). Stations GPS coordinates are provided in Table S3.
DNA extraction
eDNA was extracted from one half filter with the DNeasy® Blood and Tissue extraction kit with minor modifications to the manufacturer’s instructions, i.e. filters were digested for 2 h at 56 °C in a mixture of 756 µL AL buffer and 84 µL of proteinase K. The elution step of the DNA extraction protocol was modified; the solution was incubated for five minutes using a TRIS buffer (10 mM) instead of the elution with the AE buffer containing EDTA, that likely inhibits the qPCR reaction (QIAGEN 2017). The elution volume was 80 µL. An extraction negative control, consisting in a half filter immersed in Milli-Q® water, was included with the samples. DNA extracts were preserved at -20 °C until qPCR detection and at -80 °C for long-term preservation.
Inhibition qPCR assay
We used an internal positive control (IPC), to assess for qPCR inhibition in sample DNA extract, following the protocol developed by Chevrinais et al. (2023). We added 220,000 copies of the IPC plasmid to all qPCR replicates from samples and field negative control. Inhibition was present in the sample DNA extract if the Cq value was delayed by 2 or more cycles compared to that of the DNA extract from the field negative control (LeBlanc et al. 2020).
Specific qPCR assay
We tested each DNA extract with three qPCR replicates and the A. lupus targeted qPCR assay (optimized qPCR protocol described in “In vitro optimization”). Each qPCR plate contained one to five qPCR negative controls, and one qPCR positive control. The qPCR positive control consisted in a concentration of 2,000 copies of a plasmid, including the partial CYTB gene of A. lupus and an insertion of 6-nucleotides. This insertion of 6-nucleotides would enable to identify cross-contamination during qPCR plate preparation. Replicates of qPCR from sample DNA extracts were considered positive if they showed a sigmoidal amplification curve and a Cq ≥ LOD95% (Fig. S2).
All qPCR positive detections were Sanger sequenced to confirm the specificity of the A. lupus qPCR assay and the absence of contamination from the qPCR positive control. Products of qPCR were amplified using 1 µL of amplicon, 2 µL of each primer (10 µM), 10 µL of 10% trehalose and 0.5 µL of the Big Dye Terminator, 3.75 µL of the 5x buffer from the Applied Biosystems BigDye™ Terminator v3.1 cycle sequencing kit (Thermo Fischer Scientific, MA, USA) and 3.88 µl of nuclease-free water to yield a 20 µL final reaction. Cycling parameters were 1 min. denaturation step at 96 °C followed by 25 cycles consisting of a 10 s. denaturation step at 96 °C, a 5 s. annealing step at 50 °C and a 4 min. elongation step at 60 °C. Following amplification, PCR products were cleaned using the Applied Biosystems BigDye® Xterminator™ purification kit (Thermo Fischer Scientific, MA, USA) and sequenced on the Applied Biosystems SeqStudio genetic analyzer system (Thermo Fischer Scientific, MA, USA).
The qPCR negative controls were considered contaminated if a sigmoidal amplification curve was observed. A qPCR positive control failed if the expected Cq value was delayed by 2 or more cycles than its Cq value in the standard curve.
Criteria for reporting eDNA results from the qPCR assay
Environmental DNA detection results were reported for each of the three qPCR replicates per sample. We also used a decision tree with minimum criteria to summarize eDNA detection results at the sample and station levels. The minimum criteria for positive samples was that at least two out of three qPCR replicates showed an amplification curve and DNA copies number ≥ LOD95%. A sample was considered as inconclusive if one out of three qPCR replicates showed an amplification curve and DNA copies number ≥ LOD95%. A sample was considered as (1) negative if no amplification curves was observed or as (2) inconclusive if DNA copies < LOD95% were detected in all three qPCR replicates. A station was identified as positive if at least one out of the two samples (at 15 m or bottom) was positive, as inconclusive if at least one sample was inconclusive, and as negative if no sample was positive or inconclusive.
Results
qPCR assay performance
The qPCR assay was specific to A. lupus (Fig. S2). The in vitro specificity testing of qPCR assay for A. lupus showed no amplification of closely related species belonging to the Anarhichas genus (Fig. S2). The LOD95% was of 1.5 copies per qPCR reaction (Table 1).
In situ validation
Negative and positive controls used at various steps of the protocol passed for the A. lupus qPCR assay (Fig. S3). Inhibition was not detected in all samples collected in this study, i.e., the IPC assay showed no delayed amplification compared to their field negative control (Fig. S4).
Anarhichas lupus DNA was detected in qPCR replicates from 15 m and bottom samples. The number of copies detected varied between 5 (15 m, station 5) and 16 (bottom, station 3) across positive qPCR replicates (Fig. S5). All qPCR positive replicates were sequenced, which confirmed that the target species was detected and that no contamination from the qPCR positive control occurred.
For bottom samples, station 1 had two positive qPCR replicates, stations 2, 3, 4, and 6 had one positive qPCR replicate, and station 5 had no positive qPCR replicates (Fig. 1). Bottom sample from station 1 was positive whereas those from stations 2, 3, 4, and 6 were inconclusive. For 15 m samples, station 5 had one positive qPCR replicate, all other stations had no positive qPCR replicates. Therefore, 15 m sample from station 5 was inconclusive and all other samples were negative. At the station level, station 1 was positive and all other stations were inconclusive for A. lupus eDNA detections.
Discussion
This study provides a specific and sensitive qPCR assay to detect A. lupus eDNA. This new powerful tool can be used in trawlable and untrawlable areas for the detection of A. lupus eDNA and limit the mortality of this species associated with ongoing survey activities. Future improvements to the A. lupus eDNA assay and future studies are discussed.
qPCR assay performance
The A. lupus qPCR assay and eDNA protocol were validated to essential-level 3 according minimum criteria from Abbott et al. (2021) and Thalinger et al. (2021). This validation level implies that a qPCR assay and eDNA detection protocol went through in vitro specificity and sensitivity testing, and in situ testing. At the level 3, results should be interpreted as eDNA detections indicate that the target is likely present whereas the absence of eDNA detections cannot provide a conclusion whether the target is present or not (Abbott et al. 2021; Thalinger et al. 2021). We also established the LOD95%, a criteria needed for substantial-level 4 of validation (Abbott et al. 2021; Thalinger et al. 2021), at 1.5 copies per qPCR reaction which is adapted to rare species detection and falls into the lower range of the literature (Klymus et al. 2019). This LOD95% could also be improved by increasing the number of qPCR replicates in the standard curve and using binomial detections (Lesperance et al. 2021).
eDNA detections in situ
We detected Atlantic wolffish eDNA in qPCR replicates in samples from all six stations in the Gulf of St. Lawrence where the species had been captured in the trawl survey. The majority of qPCR positive detections was from bottom samples which coincide with high densities of juveniles or adults A. lupus at depths between 80 and 350 m (Kulka et al. 2007; Bourdages and Ouellet 2011; Dutil et al. 2014). Maximum densities of Atlantic wolffish with trawl data were also observed in the west coast of Newfoundland during the 2020 DFO annual ecosystem survey (Bourdages et al. 2021). The eDNA of A. lupus was also detected in shallow waters between 15 and 115 m (stations 1, 3, 4, 5, Table S3). Detections of eDNA in shallow waters are also expected but at lower densities for adults (Novaczek et al. 2017) or during their seasonal migration for spawning (Keats et al. 1986; Nelson and Ross 1992).
Station 1 was positive whereas all other stations were inconclusive for A. lupus eDNA detection. This result suggests that a greater number of qPCR replicates should be used to investigate for the presence of rare A. lupus eDNA. Theoretically a higher number of replicates would imply lower limits of detection and quantification which could allow an increased sensitivity for the detection of rare eDNA (see Figs 3 and 4 in Lesperance et al. 2021). Higher number of replicates both in the field and in the laboratory (e.g., qPCR replicates) and the sampling of larger volumes would also increase the capacity to detect eDNA, especially for low-abundance species (Sepulveda et al. 2019; Schabacker et al. 2020; Buxton et al. 2021; Govindarajan et al. 2022).
This study provides a monitoring tool for A. lupus to survey untrawlable areas (e.g., shoals). Such tool would also be valuable for the two other Anarhichas species occurring in the northwest Atlantic Ocean that are also Species-At-Risk. Future studies should also assess the quantitative performance of the A. lupus eDNA assay. This would necessitate a greater number of samples and a direct comparison between eDNA concentration to trawl captures of A. lupus across a study area.
Data Availability
“The datasets generated during and/or analysed during the current study are available from the corresponding author on request.”
References
Abbott C, Coulson M, Gagné N et al (2021) Guidance on the use of targeted environmental DNA (eDNA) analysis for the management of aquatic invasive species and species at risk.DFO Can Sci Advis Sec Res Doc2021/019:iv + 42 p
Antognazza CM, Britton JR, Potter C et al (2019) Environmental DNA as a non-invasive sampling tool to detect the spawning distribution of european anadromous shads (Alosa spp). Aquat Conserv Mar Freshw Ecosyst 29:148–152. https://doi.org/10.1002/aqc.3010
Beng KC, Corlett RT (2020) Applications of environmental DNA (eDNA) in ecology and conservation: opportunities, challenges and prospects. Biodivers Conserv 29:2089–2121. https://doi.org/10.1007/s10531-020-01980-0
Bluemel JK, Fischer SH, Kulka DW et al (2022) Decline in Atlantic wolffish Anarhichas lupus in the North Sea: impacts of fishing pressure and climate change. J Fish Biol 100:253–267. https://doi.org/10.1111/jfb.14942
Bourdages H, Ouellet JF (2011) Geographic distribution and abundance indices of marine fish in the northern Gulf of St. Lawrence (1990–2009). Can Tech Rep Fish Aquat Sci 2963:171
Bourdages H, Brassard C, Desgagnés M et al (2021) Preliminary results from the ecosystemic survey in August 2020 in the Estuary and northern Gulf of St. Lawrence.DFO Can Sci Advis Sec Res Doc2021/054:iv + 93 p
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Buxton A, Matechou E, Griffin J et al (2021) Optimising sampling and analysis protocols in environmental DNA studies. Sci Rep 11:11637. https://doi.org/10.1038/s41598-021-91166-7
Chevrinais M, Demers A, Dumoulin L-A et al (2023) Targeted detection of european green crab (carcinus maenas) and chinese mitten crab (eriocheir sinensis) using environmental DNA. Can Tech Rep Fish Aquat Sci 3539:vii
Darling JA (2019) How to learn to stop worrying and love environmental DNA monitoring. Aquat Ecosyst Health Manag 22:440–451. https://doi.org/10.1080/14634988.2019.1682912
Davy CM, Kidd AG, Wilson CC (2015) Development and validation of environmental DNA (eDNA) markers for detection of freshwater turtles. PLoS ONE 10:e0130965. https://doi.org/10.1371/journal.pone.0130965
Dutil J-D, Proulx S, Chouinard P-M et al (2014) Distribution and environmental relationships of three species of wolffish (Anarhichas spp.) in the Gulf of St. Lawrence. Aquat Conserv Mar Freshw Ecosyst 24:351–368. https://doi.org/10.1002/aqc.2370
Gagne N, Bernatchez L, Bright D et al (2021) Environmental DNA (eDNA) reporting requirements and terminology. Canadian Standards Association
Gargan LM, Morato T, Pham CK et al (2017) Development of a sensitive detection method to survey pelagic biodiversity using eDNA and quantitative PCR: a case study of devil ray at seamounts. Mar Biol 164:112. https://doi.org/10.1007/s00227-017-3141-x
Goldberg CS, Turner CR, Deiner K et al (2016) Critical considerations for the application of environmental DNA methods to detect aquatic species. Methods Ecol Evol 7:1299–1307. https://doi.org/10.1111/2041-210X.12595
Govindarajan AF, McCartin L, Adams A et al (2022) Improved biodiversity detection using a large-volume environmental DNA sampler with in situ filtration and implications for marine eDNA sampling strategies. Deep Sea Res Part I Oceanogr Res Pap 189:103871. https://doi.org/10.1016/j.dsr.2022.103871
Hansen BK, Jacobsen MW, Middelboe AL et al (2020) Remote, autonomous real-time monitoring of environmental DNA from commercial fish. Sci Rep 10:13272. https://doi.org/10.1038/s41598-020-70206-8
Jo T, Takao K, Minamoto T (2022) Linking the state of environmental DNA to its application for biomonitoring and stock assessment: targeting mitochondrial/nuclear genes, and different DNA fragment lengths and particle sizes. Environ DNA 4:271–283
Johnstone KA, Marshall HD, Carr SM (2007) Biodiversity genomics for species at risk: patterns of DNA sequence variation within and among complete mitochondrial genomes of three species of wolffish (Anarhichas spp). Can J Zool 85:151–158. https://doi.org/10.1139/z06-191
Keats DW, South GR, Steele DH (1986) Where do juvenile atlantic wolffish, Anarhichas lupus, live? Can field-naturalist Ottawa 100:556–558
Keller AG, Grason EW, McDonald PS et al (2022) Tracking an invasion front with environmental DNA. Ecol Appl e2561
Klymus KE, Merkes CM, Allison MJ et al (2019) Reporting the limits of detection and quantification for environmental DNA assays. Environ DNA 2:271–289. https://doi.org/10.1002/edn3.29
Kulka DW, DeBlois EM (1996) Non traditional Groundfish Species on Labrador Shelf and Grand Banks - Wolffish, Monkfish, White Hake and Winter (blackback) flounder. DFO Can Sci Advis Sec Res Doc 097:49
Kulka D, Hood C, Huntington J (2007) Recovery Strategy for Northern Wolffish (Anarhichas denticulatus) and spotted wolffish (Anarhichas minor), and Management Plan for Atlantic Wolffish (Anarhichas lupus) in Canada. Fish Ocean Canada Newfounl Labrador Reg 103
Lait LA, Carr SM (2018) Intraspecific mitogenomics of three marine species-at-risk: Atlantic, spotted, and northern wolffish (Anarhichas spp). Genome 61:625–634. https://doi.org/10.1139/gen-2018-0043
Leempoel K, Hebert T, Hadly EA (2020) A comparison of eDNA to camera trapping for assessment of terrestrial mammal diversity. Proc R Soc B Biol Sci 287:20192353. https://doi.org/10.1098/rspb.2019.2353
LeBlanc F, B?lliveau V, Watson E, Coomber C, Simard N, DiBacco C, Bernier R, and Gagn? N (2020). Environmental DNA (eDNA) detection of marine aquatic invasive species (AIS) in Eastern Canada using a targeted species-specific qPCR approach. Manag Biol Invasions 11(2): 201
Lesperance ML, Allison MJ, Bergman LC et al (2021) A statistical model for calibration and computation of detection and quantification limits for low copy number environmental DNA samples. Environ DNA 3:970–981. https://doi.org/10.1002/edn3.220
McCallum B, Walsh SJ (2002) An update on the performance of the Campelen 1800 during bottom trawl surveys in NAFO Subareas 2 and 3 in 2001. NAFO SCR Doc 2:16
McRuer JK, Hurlbut T, Morin B (2000) Status of Atlantic wolffish (Anarhichas lupus) in the Maritimes (NAFO Sub-Area 4 and 5). DFO Can Sci Advis Sec Res Doc 138:57
Moran AJ, Prosser SWJ, Moran JA (2019) DNA metabarcoding allows non-invasive identification of arthropod prey provisioned to nestling Rufous hummingbirds (Selasphorus rufus). PeerJ 7:e6596. https://doi.org/10.7717/peerj.6596
Nelson GA, Ross MR (1992) Distribution, growth and food habits of the Atlantic wolffish (Anarhichas lupus) from the Gulf of Maine-Georges Bank region. J Northw Atl Fish Sci 13:53–61. https://doi.org/10.2960/J.v13.a4
Novaczek E, Devillers R, Edinger E, Mello L (2017) High-resolution seafloor mapping to describe coastal denning habitat of a canadian species at risk: Atlantic wolffish (Anarhichas lupus). Can J Fish Aquat Sci 74:2073–2084. https://doi.org/10.1139/cjfas-2016-0414
QIAGEN (2017) DNeasy PowerWater kit Handbook
Rees HC, Maddison BC, Middleditch DJ et al (2014) The detection of aquatic animal species using environmental DNA–a review of eDNA as a survey tool in ecology. J Appl Ecol 51:1450–1459. https://doi.org/10.1111/1365-2664.12306
Roux LD, Giblot-Ducray D, Bott NJ et al (2020) Analytical validation and field testing of a specific qPCR assay for environmental DNA detection of invasive european green crab (Carcinus maenas). Environ DNA 2:309–320. https://doi.org/10.1002/edn3.65
Schabacker JC, Amish SJ, Ellis BK et al (2020) Increased eDNA detection sensitivity using a novel high-volume water sampling method. Environ DNA 2:244–251. https://doi.org/10.1002/edn3.63
Sepulveda AJ, Schabacker J, Smith S et al (2019) Improved detection of rare, endangered and invasive trout in using a new large-volume sampling method for eDNA capture. Environ DNA 1:227–237. https://doi.org/10.1002/edn3.23
Simpson MR, Mello LGS, Miri CM et al (2014) A preliminary analysis of habitat use and movement patterns of wolffish (anarhichas spp.) in Coastal Newfoundland Waters. DFO Can Sci Advis Sec Res Doc 033:v
Stoeckle MY, Das Mishu M, Charlop-Powers Z (2018) GoFish: a versatile nested PCR strategy for environmental DNA assays for marine vertebrates. PLoS ONE 13:e0198717. https://doi.org/10.1371/journal.pone.0198717
Templeman W (1984) Migrations of wolffishes, Anarhichas sp., from tagging in the Newfoundland area. J Northwest Atl Fish Sci 5:93–97. https://doi.org/10.2960/J.v5.a11
Thalinger B, Deiner K, Harper LR et al (2021) A validation scale to determine the readiness of environmental DNA assays for routine species monitoring. Environ DNA 3:823–836. https://doi.org/10.1002/edn3.189
Thomsen PF, Kielgast JOS, Iversen LL et al (2012) Monitoring endangered freshwater biodiversity using environmental DNA. Mol Ecol 21:2565–2573. https://doi.org/10.1111/j.1365-294X.2011.05418.x
Veldhoen N, Hobbs J, Ikonomou G et al (2016) Implementation of novel design features for qpcr-based eDNA assessment. PLoS ONE 11:e0164907. https://doi.org/10.1371/journal.pone.0164907
Weltz K, Lyle JM, Ovenden J et al (2017) Application of environmental DNA to detect an endangered marine skate species in the wild. PLoS ONE 12:e0178124. https://doi.org/10.1371/journal.pone.0178124
Wilcox TM, McKelvey KS, Young MK et al (2013) Robust detection of rare species using environmental DNA: the importance of primer specificity. PLoS ONE 8:e59520. https://doi.org/10.1371/journal.pone.0059520
Wood SA, Pochon X, Laroche O et al (2019) A comparison of droplet digital polymerase chain reaction (PCR), quantitative PCR and metabarcoding for species-specific detection in environmental DNA. Mol Ecol Resour 19:1407–1419. https://doi.org/10.1111/1755-0998.13055
Acknowledgements
We are grateful to Éric Parent, Laury-Ann Dumoulin, Jade Larivière, and Ariane Therien from the Laboratory of Genomics for their technical support during the project. We would also like to thank Geneviève Faille and Geneviève Côté from the DFO Marine Conservation Target and all members of the DFO eDNA working group for insightful discussions. We thank Steven M Carr and an anonymous reviewer for their constructive comments that greatly improved the manuscript.
Funding
Open access funding provided by Fisheries & Oceans Canada
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Marion Chevrinais. The first draft of the manuscript was written by Marion Chevrinais. Geneviève J. Parent commented and improved previous versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chevrinais, M., Parent, G.J. A validated targeted assay for environmental DNA detections of the Atlantic wolffish (Anarhichas lupus). Conservation Genet Resour 15, 59–66 (2023). https://doi.org/10.1007/s12686-023-01302-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12686-023-01302-w