Abstract
In addition to harming the respiratory system, COVID-19 can affect multiple organs. Children may develop a specific complication of COVID-19 called multisystem inflammatory syndrome in children (MIS-C) which could influence the vascular system of children and cause multiple coagulopathies in the body. Information on the use of thromboprophylaxis in this condition was collected via the review of various articles. In general, different factors in immune system responses can trigger the initiation of thrombotic events. Studies have shown that starting anticoagulant prophylaxis, which contributes to decreased thrombotic events, is dependent on the patient's condition and D-dimer levels. However, further studies on pediatric populations are needed to establish the role of anticoagulants in children with this condition.
Similar content being viewed by others
Utilization of low-molecular-weight heparin (enoxaparin) prophylaxis for COVID-19 pediatric patients could be a safe practice but it is not suggested for all cases. Enoxaparin as thromboprophylaxis may be considered depending on case condition and presence of risk factors such as high D-dimer levels, or presence of multisystem inflammatory syndrome in children (MIS-C) criteria. |
Extended thromboprophylaxis after discharge was not recommended in pediatric patients due to limited evidence, but should be reviewed case by case. Multiple risk factors should be considered before considering post-discharge thromboprophylaxis. |
1 Introduction
Children may develop a special complication of Coronavirus Disease 2019 (COVID-19), known as multisystem inflammatory syndrome in children (MIS-C), which could cause vascular damage alongside multiple organ involvement. One of the consequences of MIS-C is thrombosis and development of thrombotic events has been associated with worse outcomes [1, 2]. In the current review, MIS-C and thrombosis interplay and suggested prophylactic anticoagulants are discussed.
2 Multisystem Inflammatory Syndrome in Children (MIS-C) Related to COVID-19
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related COVID-19 is one of the most life-threatening epidemics in the world after the ‘Spanish flu’ pandemic in 1918, causing devastating consequences for both human health and socioeconomic welfare. Since the widespread occurrence of COVID-19 in 2019, over 700 million people have been infected and over 6.8 million people died during the pandemic, while many countries experienced economic losses as well [3]. The results of a myriad of clinical observations and trials were reported, all striving to better inform, manage and treat the disease. Evidence shows that COVID-19 disease is not merely an isolated respiratory infection; it causes development of a multisystem inflammatory syndrome and results in complications to patients, mandating a multi-disciplinary approach. A SARS-CoV-2-related inflammatory syndrome, MIS-C, may develop in children, usually weeks after acute infection. MIS-C is likely to present with arrhythmias, coronary artery aneurysms, myocarditis, and sudden cardiogenic shock; all of which tend to evolve rapidly [4]. Despite the unknown cause of MIS-C, scientists have suggested that a dysfunctional immune response results in cytokine release and organ damage [5]. From this perspective, MIS-C was contrasted with and mimicked Kawasaki disease, cytokine release syndrome, and other autoimmune disorders.
MIS-C can cause changes in blood parameters. A study has shown that the increase in C-reactive protein (CRP), ferritin, D-dimer, white blood cell (WBC) count, along with rotational thromboelastometry (ROTEM) factors in MIS-C patients are connected to a hypercoagulable state [6] caused by endothelial damage in the setting of hyperinflammation, leading to coagulation abnormalities and microvascular alongside macrovascular thrombosis in these patients [1, 7, 8]. MIS-C brings about a thrombosis risk of approximately 3.5% [9] (see Table 1).
3 Pathogenesis of Thrombosis
As an integral component of the SARS-CoV-2-related disease pathogenesis, coagulopathy presents a predictor of poor prognosis [4]. Deep venous thrombosis of the limbs and other sites, microvascular pulmonary thrombosis, and macrovascular pulmonary artery thrombosis/embolism can occur in patients with coronavirus infections [10, 11]. The pathophysiology of COVID-19-related thrombotic abnormalities can be described using Virchow's triad: endothelial damage, stasis of blood flow, and coagulopathy [12]. A study that was conducted in pediatric patients revealed that the rates of symptomatic venous thromboembolism (VTE) were 7% and 1.3% among patients 13–21 years old and 5–13 years old, respectively [7]. In another study, which was conducted in adult COVID-19 patients with median age of 65 years (age range: 36–80 years), pulmonary embolism (PE) and deep vein thrombosis (DVT) incidence rates were 16.5% and 14.8%, respectively [10]. In a comparison between pediatrics and the adult patient population, the incidence of thrombosis is higher in the second group. Based on a report from the International Society on Thrombosis and Hemostasis (ISTH), around 71.4% of adult COVID-19 non-survivors, mean age 70 years old, exhibited disseminated intravascular coagulation (DIC) [11].
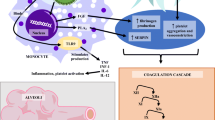
SARS-CoV-2 infects cells via the type 2 angiotensin-converting enzyme receptor (ACE2). Numerous human cell types express this receptor type, including endothelial cells (EC), podocytes, cardiac myocytes, and alveolar cells. Therefore, SARS-CoV-2 directly affects the vascular system, comprising veins and arteries. Damage to EC leads to release of the tissue factors, activating the coagulation cascade, initiating the complement system, and the start of an inflammatory system response known as a ‘cytokine storm’ [12]. Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are drastically elevated in patients with severe COVID-19. Through an encounter with EC, both of the abovementioned cytokines exert prothrombotic characteristics [13, 14]. Besides, IL-6 elevates inflammatory responses more than other factors, capable of developing thrombotic events [15]. Moreover, TNF-α provokes the complement system, in turn stimulating the coagulation system [16]. In patients with severe COVID-19-related systemic inflammation, several markers represent the imbalance between coagulation and fibrinolysis, which have similar features to cytokine storm or macrophage activation syndrome [17]. An intravascular coagulopathy with significant hemorrhagic infarction, vessel wall edema, and capillary thrombosis arises from systemic EC damage at the level of the respiratory system, serving as the entry point for COVID-19 infection. Further systemic thromboses and emboli, particularly sizable arterial thromboses, can develop once the pathogen damages EC in other areas [15]. Pathophysiology of thrombosis development is shown in Fig. 1. As a result, the dysfunction of the antithrombotic and anti-inflammatory system likely is the prominent cause of coagulopathy in Covid-19 settings, alongside other complications. Zhang et al. reported that the level of anticoagulant proteins like antithrombin, protein S, and protein C is decreased in COVID-19 adult patients [10]. The severe inflammatory state, known as DIC, which is mainly detected in sepsis, causes activated partial thromboplastin time (aPTT) and prothrombin time (PT) as well as the D-dimer levels to rise, as reported in sepsis and COVID-19 pediatric patients [18].
Flowchart showing a protocol for evaluation and thromboprophylaxis in children hospitalized with COVID-19. ARDS acute respiratory distress syndrome, CRRT continuous renal replacement therapy, ECMO extracorporeal membrane oxygenation, ICU intensive care unit, LMWH low molecular weight heparin, UFH unfractionated heparin, VTE venous thromboembolism
4 Risk Factors for Thrombosis
Assessing the severity of COVID-19 infection is a crucial first step in determining the risk of thrombosis. Among adult patients, worsening conditions and higher oxygen needs are related to worse outcomes, including thrombosis [19, 20]. About 95% of children with acute infections show milder clinical manifestations than adults, and many recover without problems. Nevertheless, like adults, respiratory symptoms and increased oxygen requirements are associated with higher risk of VTE in children [21].
According to a study by Whitworth et al., the occurrence rate of venous thromboembolism in patients with MIS-C was higher than that in those with COVID-19. In the MIS-C group, the incidence rate was 6.5% and it was associated with increased fibrinogen and D-dimer levels, and presence of thromboembolic risk factors such as cancer, central venous catheter, or age older than 12 years [1]. Although thromboprophylaxis in non-critically ill children can reduce thromboembolic incidents, in critically ill children, defined as pediatric patients hospitalized with MIS-C or severe COVID-19, despite receipt of thromboprophylaxis, the rate of thromboembolic events was high (more than two third of thromboembolisms were from patients who received thromboprophylaxis). However, thrombotic events were lower in patients under 12 years old [1].
The American Society of Hematology (ASH), the Society of Critical Care Medicine (SCCM), and the American Academy of Pediatrics (AAP) have not yet released guidelines or recommendations for thromboprophylaxis in hospitalized children with MIS-C. However, ISTH suggests thromboprophylaxis for children hospitalized with COVID-19, including those with MIS-C, with significantly increased D-dimer levels (≥5 times the upper limit of normal values) or coexisting clinical risk factors (presence of central venous catheter, obesity, flare of underlying inflammatory disease, previous history of VTE, first-degree family history of VTE before age 40 years or unprovoked VTE, known thrombophilia, receiving estrogen-containing oral contraceptive pill, or need for mechanical ventilation, etc.) based on the expert opinion [2]. Due to limited available data regarding pediatrics, adopting thromboprophylaxis techniques in children with COVID-19 is challenging.
5 Laboratory Findings of the Coagulopathy
Based on many reports, coagulation factors like D-dimer levels are dependent on age during infancy and childhood, underscoring the necessity of age-specific reference ranges in the accurate evaluation and management of thrombotic incidents in pediatric populations, and this makes these findings less reliable in making a diagnosis [20]. The abnormalities identified in the extensive coagulation such as elevated levels of D-dimer and fibrin degradation products, aPTT, PT, lower antithrombin levels, higher activity of von Willebrand factor and factor VIII, and thrombocytopenia were ascribed to predict poor prognosis and not diagnosis or risk evaluation in adults and pediatrics with COVID-19, therefore D-dimer levels may be considered a weak option as a factor guiding therapeutic decision making regarding anticoagulant prophylaxis [4, 21].
Increased levels of inflammatory markers (e.g., D-dimer), lack of thromboprophylaxis, and the presence of risk factors for clinically relevant bleeding caused by epitheliopathy, platelet dysfunction, and consumptive coagulopathy in COVID-19 patients are imperative to discover favorable thromboprophylaxis strategies. Pediatric physicians face more challenges in incorporating available evidence to apply thromboprophylaxis strategies in children with COVID-19, because the incidence of severe COVID-19 in children is lower compared with adults and for this reason, less investigation has been carried out about pediatric thromboprophylaxis in this situation.
6 Thromboprophylaxis
Enoxaparin was approved in 1993 [22]. It is a low molecular weight heparin (LMWH) with a mean molecular weight of 4000 to 5000 Daltons. The indications for use of enoxaparin are thrombotic events such as treatment of VTE or PE, acute coronary syndromes, and DVT prophylaxis in different circumstances; the medication prevents the formation of blood clots by binding to antithrombin III, forming a complex that irreversibly inactivates factor Xa [23].
Although an investigation showed that enoxaparin injection twice per day, with initial dose of 0.5 mg/kg per dose (maximum 60 mg per dose) in patients under 18 years old (median age 12.1 years [range 1.3–17.5]), to achieve the target anti-Xa activity of 0.20–0.49 ng/mL, was safe and efficient in hospitalized children with COVID-19, without increased risk of life-threatening adverse effects or bleeding [24]. Prescription of anticoagulant thromboprophylaxis is not regularly carried out in children hospitalized with asymptomatic SARS-CoV-2 infection in the absence of multiple clinical risk factors for hospital-related VTE. In this clinical trial, the median dose for achieving goal levels of anti-Xa was 0.5 mg/kg/dose and it was not significantly different in various age ranges (<12 years old or >12 years old). It was also shown that the median dose was significantly higher in patients with MIS-C. Two studies have illustrated that thromboprophylaxis may not be effective based on the incidence of thrombotic complications in spite of thromboprophylaxis. One of these studies revealed that about 70% of pediatric patients experienced thrombotic complications in spite of receiving prophylactic anticoagulation [1]. Similarly, based on the results of another study, VTE occurred in more than 30% of children [8]. This study was conducted in children with an age range of 2 months to 21 years, and only patients hospitalized with symptomatic COVID-19 were included. Six of ten patients who received LMWH and had their dose adjusted to anti-Xa levels of 0.2–0.4 ng/mL were not diagnosed with VTE, while three of the four patients who were on a fixed dose of LMWH, either 40 mg daily or a weight-based fixed dose, were diagnosed with VTE [8]. The outcomes of the study showed that in spite of thromboprophylaxis, the occurrence rates of thrombotic cases might be high [8]. These results also suggest that therapeutic dosing of LMWH might provide superior effects in thromboprophylaxis; however, results from high quality studies are needed to confirm both the safety and efficacy of therapeutic over prophylactic dosing in children.
In the study conducted by Del Borrello et al., it was shown that although widespread anticoagulant prophylaxis is not recommended for hospitalized children with COVID-19, 10 U/kg/h of unfractionated heparin (UFH) or 100 U/kg/24h (equivalent to 1 mg/kg/24h) of enoxaparin, may be prescribed for carefully selected patients with multiple risk factors for coagulopathy (the authors used an institutional risk assessment model that included cardiovascular comorbidities, patient’s mobility, and risk of bleeding), regardless of D-dimer levels [25]. Similarly, another study confirmed that the normalization of irregularities occurred shortly after admission and thromboprophylaxis did not appear to be beneficial [26]. However, this study concluded that coagulopathy may not be related to the rates of VTE and the pediatric patients who were hospitalized with severe COVID-19 should be evaluated individually. Several risk factors should be investigated in order to prescribe thromboprophylaxis. Considering the degree of morbidity related to healthcare-associated VTE, protocols have been defined for thromboprophylaxis in hospitalized pediatric patients, including assessments specific to the pediatric intensive care unit setting and analyses are done on moderately ill, hospitalized children [27]. Loi et al. recommended LMWH thromboprophylaxis in COVID-19 patients presenting with increased D-dimer levels, increased fibrinogen levels, DIC, or average or mildly decreased platelet count, and for the children with risk factors of developing VTE [28]. This protocol is shown in Fig. 2.
Flowchart showing suggested mechanism of thrombosis in patients with severe COVID-19. CRP C-reactive protein, DAMPs danger-associated molecular patterns, ICAM intercellular adhesion molecule, IL interleukin, PAI-1 plasminogen activator inhibitor-1, TF tissue factor, TNFα tumor necrosis factor alpha, tPA tissue-type plasminogen activator, VCAM vascular cell adhesion molecule, VWF Von Willebrand factor
In a recent study, the results did not show significant effects of thromboprophylaxis on mortality in pediatric patients with COVID-19, but enoxaparin showed a negligible effect on mortality rate (p-value: 0.57) [29]. The investigators suggested that, despite the results of the study, thromboprophylaxis could be helpful in pediatric patients with moderate to severe COVID-19 classified based on the World Health Organization (WHO) progression scale; further randomized clinical trials with larger sample sizes are needed to prove this conclusion. In a recent cohort study that evaluated thromboprophylaxis in patients with MIS-C, it was demonstrated that thromboprophylaxis is useful in these patients after first considering multiple factors including rising laboratory parameters such as D-dimer levels [30]. It is imperative to consider clinical parameters such as illness severity and VTE risk factors prior to thromboprophylaxis administration and determining the dose of the agent. Using the tailored-intensity thromboprophylaxis, which adjusts the dose of enoxaparin to a specific level of anti-Xa based on the patient's condition, is another crucial factor. The studies have shown that the median dose of LMWH for prophylaxis was 0.5 mg/kg/dose twice daily and for therapeutic intensity was 1.0 mg/kg/dose twice daily [30].
A practical guideline provided by Karimi et al., states that patients with D-dimer levels of over 300 ng/mL should receive LMWH prophylaxis and be evaluated for DVT, but any irregularity in aPTT, PT, platelet count, fibrinogen, and D-dimer should be considered and followed. Furthermore, the guideline suggests that a high index of speculation for thrombosis should be maintained. It also recommends that pediatric patients with moderate COVID-19 (patients with fever, respiratory symptoms, and radiographical features) who need hospitalization should receive prophylactic LMWH anticoagulation [31]. In children with severe COVID-19, it is suggested to intensify anticoagulation therapy in combination with ultrasonography screening if D-dimer and serum ferritin levels are > 500 ng/mL, and if the patient’s situation deteriorates [31].
Recent updated clinical guidance suggests thromboprophylaxis with LMWH twice daily (enoxaparin 0.5 mg/kg targeted to an anti-Xa activity level of 0.2 to < 0.5 IU/mL) in acute COVID-19 or MISC patients with risk factor for VTE or a significant rise in their D-dimer levels [32]. This guidance also recommends against the use of thromboprophylaxis in asymptomatic COVID-19-infected patients without any risk factor for VTE development.
In general, LMWH is preferred to UFH in pediatric patients due to good bioavailability, long duration of action and more studies supporting its use in the pediatric patient population [33]. The phase II trial, which was conducted in pediatric COVID-19 and MIS-C patients in order to investigate safety and optimal dose of enoxaparin, showed that based on the absence of clinically relevant bleeding events and other adverse effects within the prespecified range of dose, this medication is safe [24]. However, the results of the study showed that dose of enoxaparin is not significantly different between patients under or above 12 years of age. This study also showed that the enoxaparin dosing which was used in MIS-C patients is higher than that for other pediatric COVID-19-infected patients to achieve the target of anti-Xa [24]. The initial prophylactic dose of enoxaparin in this clinical trial and others was 0.5 mg/kg twice daily for patients over 2 months of age [24, 30, 32], which was in correlation with the recommendations from the Chest guideline regarding the general preventive dose of enoxaparin for thromboprophylaxis in pediatric patients (age-dependent dosing: < 2 months: 0.75 mg/kg per dose twice daily and ≥ 2 months: 0.5 mg/kg per dose twice daily) [34].
Another medication that is used in the anticoagulation protocol in addition to LMWH is aspirin. Aspirin inhibits platelet activity by irreversible inhibition of the cyclooxygenase (COX) function. COVID-19 causes lung damage which can induce thrombotic events, and antithrombotic drugs decrease coagulopathy accidents [35]. The American College of Rheumatology guidelines recommend the following regarding aspirin in MIS-C patients: low-dose aspirin (3–5 mg/kg/day; maximum 81 mg/day) should be ordered in MIS-C patients with Kawasaki disease features, coronary artery changes, or risk factors of thrombosis [36]. One possible reason for this might be the beneficial effects of aspirin in managing coronary artery aneurysms in pediatric patients with Kawasaki disease, although higher doses are used in the initial management phase of those patients [37]. This antiplatelet therapy should be continued for at least 4 weeks and until platelet counts normalize.
7 Follow Up After Discharge
After discharge, patients may need more care and follow-up to determine the level of D-dimer and risk of thrombosis. D-dimer levels five times higher than the upper limit of normal are related to thrombosis in children [33]. D-dimer levels in combination with disease severity could be useful in determining the duration of thromboprophylaxis [30]. A consensus guideline has recommended that continuing pharmacological thromboprophylaxis is required in pediatric patients with COVID-19 or MIS-C with elevated D-dimer levels or VTE risk factors after discharge [2]. The duration of anticoagulation in the post-discharge setting was recommended to be 30 days or sooner if clinical risk factors resolve.
A recent guidance generally has recommended that thromboprophylaxis should not be continued typically after discharge from the hospital in all hospitalized patients with COVID-19, even in the patients who received therapeutic intensity anticoagulation during hospitalization for thromboprophylaxis [32], but the guidance has suggested thromboprophylaxis with rivaroxaban 10 mg daily for 35 days following hospitalization in adult patients who are at increased risk of thromboembolism. Also, the guidance points out that data related to post-hospital thromboprophylaxis are limited in pediatric patients and each patient should be evaluated for risk factors individually [32]. Although there are some data regarding use of direct oral anticoagulants in older children and adolescents, their use is not considered routine practice in these patients, and data is even more limited on their use in pediatric patients with COVID-19 [33].
According to recent studies, it is currently recommended to continue thromboprophylaxis for 7–14 days or until the resolution of risk factors that the patients had at the time of discharge, such as presence of central venous line, ongoing immobility, as well as elevated D-dimer levels. Combining low-dose aspirin (3–5 mg/kg per day) with thromboprophylaxis in MIS-C may increase the risk of bleeding; nevertheless, if there are no other bleeding risk factors, this is not a contraindication [28]. In addition to LMWH, low-dose aspirin is recommended in patients with MIS-C although the intention may not be for anticoagulation [38]. In one study, patients with MIS-C who received enoxaparin in combination with aspirin did not develop any thrombosis, the same finding was reported in patients without MIS-C who only received aspirin. Enoxaparin was continued following discharge or switched to apixaban. Within 2 weeks following discharge, laboratory abnormalities were corrected and no patient was diagnosed with thrombosis [39]. Although it was a retrospective study in patients who were diagnosed with MIS-C or without MIS-C under 21 years of age, the mean age of the two groups of patients were 9 years and 8.8 years, which categorize them as pediatric groups. In another study, patients who received thromboprophylaxis were compared with those who did not and no significant difference or VTE was reported [26].
Despite all the data, children with complications of COVID-19 (including MIS-C) with considerably increased levels of plasma D-dimer at hospital discharge and overlaid clinical risk factors for VTE may be candidates for continued anticoagulant thromboprophylaxis post-hospital discharge. This may be done for a planned period, such as until resolution of the clinical risk factors or 1-month post-discharge, utilizing low-dose LMWH subcutaneously two times a day or therapeutic-intensity LMWH (e.g., targeted anti-Xa activity of 0.5–1.0 U/mL) once a day, if there are no contraindications or increased risk of bleeding [2].
The latest version of the American College of Rheumatology clinical guidance for MIS-C has recommended that MIS-C patients with thrombosis or ejection fraction of <35% should receive low-dose aspirin and a therapeutic dose of anticoagulation with enoxaparin for 3 months, until resolution of thrombosis [36]. Evaluation of thrombosis should be repeated at intervals of 4–6 weeks post-diagnosis, and anticoagulation can be discontinued if the condition is resolved. This guideline suggests the minimum duration of anticoagulation for this patient population is 2 weeks after discharge [36].
8 Conclusion
Utilization of enoxaparin prophylaxis, at an initial dose of 0.5 mg/kg twice daily, may be safe and could be recommended in pediatric patients with an age range of 1–20 years who are diagnosed with MIS-C or severe COVID-19. The treatment is later adjusted based on the levels of anti-Xa. Nonetheless, thromboprophylaxis is not suggested in all pediatric patients with COVID-19 and should be utilized depending on case condition and presence of risk factors. In addition, trials with a high number of patients are required to determine the effect of thromboprophylaxis on mortality and disease prognosis.
References:
Whitworth H, Sartain SE, Kumar R, Armstrong K, Ballester L, Betensky M, et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138:190–8. https://doi.org/10.1182/blood.2020010218.
Goldenberg NA, Sochet A, Albisetti M, Biss T, Bonduel M, Jaffray J, et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19–related illness. J Thromb Haemost. 2020;18:3099–105. https://doi.org/10.1111/jth.15073.
WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 31 Jan 2023.
Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276–88. https://doi.org/10.1016/S1473-3099(20)30651-4.
Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS–CoV-2. J Clin Invest. 2020;130:5967–75. https://doi.org/10.1172/JCI140970.
Al-Ghafry M, Aygun B, Appiah-Kubi A, Vlachos A, Ostovar G, Capone C, et al. Are children with SARS-CoV-2 infection at high risk for thrombosis? Viscoelastic testing and coagulation profiles in a case series of pediatric patients. Pediatr Blood Cancer. 2020. https://doi.org/10.1002/pbc.28737.
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–46. https://doi.org/10.1056/nejmoa2021680.
Mitchell WB, Davila J, Keenan J, Jackson J, Tal A, Morrone KA, et al. Children and young adults hospitalized for severe COVID-19 exhibit thrombotic coagulopathy. Pediatr Blood Cancer. 2021;68:1–7. https://doi.org/10.1002/pbc.28975.
Suh YJ, Hong H, Ohana M, Bompard F, Revel M-P, Valle C, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298:E70-80. https://doi.org/10.1148/radiol.2020203557.
Zhang Y, Cao W, Jiang W, Xiao M, Li Y, Tang N, et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50:580–6. https://doi.org/10.1007/s11239-020-02182-9.
Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120:1230–2. https://doi.org/10.1055/s-0040-1712097.
Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. https://doi.org/10.1016/j.thromres.2019.07.013.
Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing Glomerulopathy in a patient with COVID-19. Kidney Int Reports. 2020;5:935–9. https://doi.org/10.1016/j.ekir.2020.04.002.
Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115:3–12. https://doi.org/10.1046/j.1365-2141.2001.03061.x.
Page MJ, Bester J, Pretorius E. The inflammatory effects of TNF-α and complement component 3 on coagulation. Sci Rep. 2018;8:1812. https://doi.org/10.1038/s41598-018-20220-8.
Castelli V, Cimini A, Ferri C. Cytokine storm in COVID-19: “When You Come Out of the Storm, You Won’t Be the Same Person Who Walked in.” Front Immunol. 2020;11:8–11. https://doi.org/10.3389/fimmu.2020.02132.
McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–45. https://doi.org/10.1016/S2665-9913(20)30121-1.
Aronoff SC, Hall A, Del Vecchio MT. The natural history of severe acute respiratory syndrome coronavirus 2-related multisystem inflammatory syndrome in children: a systematic review. J Pediatric Infect Dis Soc. 2020;9:746–51. https://doi.org/10.1093/jpids/piaa112.
Oudkerk M, Buller HR, Kuijpers D, van Es N, Oudkerk SF, McLoud T, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the national institute for public health of the Netherlands. Radiology. 2020;297:E216–22. https://doi.org/10.1148/radiol.2020201629.
Appel IM, Grimminck B, Geerts J, Stigter R, Cnossen MH, Beishuizen A. Age dependency of coagulation parameters during childhood and puberty. J Thromb Haemost. 2012;10:2254–63. https://doi.org/10.1111/j.1538-7836.2012.04905.x.
Biswas I, Khan GA. Coagulation disorders in covid-19: Role of toll-like receptors. J Inflamm Res. 2020;13:823–8. https://doi.org/10.2147/JIR.S271768.
Jupalli A, Iqbal AM. Enoxaparin. [Updated 2022 Sep 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539865/. Accessed 22 Jan 2023.
Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A. Pharmacology of anticoagulants used in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:15–31. https://doi.org/10.1007/s11239-015-1314-3.
Sochet AA, Morrison JM, Jaffray J, Godiwala N, Wilson HP, Thornburg CD, et al. Enoxaparin thromboprophylaxis in children hospitalized for COVID-19: a phase 2 trial. Pediatrics. 2022. https://doi.org/10.1542/peds.2022-056726.
Del Borrello G, Giraudo I, Bondone C, Denina M, Garazzino S, Linari C, et al. SARS-COV-2–associated coagulopathy and thromboembolism prophylaxis in children: A single-center observational study. J Thromb Haemost. 2021;19:522–30. https://doi.org/10.1111/jth.15216.
Noni M, Koukou DM, Tritzali M, Kanaka-Gantenbein C, Michos A, Spoulou V. Coagulation abnormalities and management in hospitalized pediatric patients with COVID-19. Pediatr Infect Dis J. 2022;41:570–4. https://doi.org/10.1097/INF.0000000000003545.
Hanson SJ, Punzalan RC, Arca MJ, Simpson P, Christensen MA, Hanson SK, et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–7. https://doi.org/10.1097/TA.0b013e31824964d1.
Loi M, Branchford B, Kim J, Self C, Nuss R. COVID-19 anticoagulation recommendations in children. Pediatr Blood Cancer. 2020;67:17–9. https://doi.org/10.1002/pbc.28485.
Karimi M, Sanaei Dashti A, Haghpanah S, Mansoori Y, Zarei T, Amanati A, et al. Thromboprophylaxis outcome in childhood SARS-Cov-2 infection: a single-center experience. J Pediatr Hematol Oncol. 2022;Publish Ah:1–6. https://doi.org/10.1097/MPH.0000000000002557.
Schmitz AH, Wood KE, Burghardt EL, Koestner BP, Wendt LH, Badheka AV, et al. Thromboprophylaxis for children hospitalized with COVID-19 and MIS-C. Res Pract Thromb Haemost. 2022;6:1–13. https://doi.org/10.1002/rth2.12780.
Karimi M, Bozorgi H, Zarei T, Bordbar M, Amanati A, Safaei A, et al. Antithrombotic prophylaxis in children and adolescent patients with sars-cov-2 (Covid-19) infection: a practical guidance for clinicians. Acta Biomed. 2020;91:1–8. https://doi.org/10.23750/abm.v91i4.10720.
Barnes GD, Burnett A, Allen A, Ansell J, Blumenstein M, Clark NP, et al. Thromboembolic prevention and anticoagulant therapy during the COVID-19 pandemic : updated clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2022. https://doi.org/10.1007/s11239-022-02643-3.
Tran VL, Parsons S, Varela CR. The trilogy of SARS-CoV-2 in pediatrics (Part 3): Thrombosis, anticoagulant, and antiplatelet considerations. J Pediatr Pharmacol Ther. 2021;26:565–76. https://doi.org/10.5863/1551-6776-26.6.565.
Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, et al. Antithrombotic therapy in neonates and children: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e737S-e801S. https://doi.org/10.1378/chest.11-2308.
Wijaya I, Andhika R, Huang I, Purwiga A, Budiman KY. The effects of aspirin on the outcome of COVID-19: A systematic review and meta-analysis. Clin Epidemiol Glob Heal. 2021;12:100883. https://doi.org/10.1016/j.cegh.2021.100883.
Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19: version 3. Arthritis Rheumatol. 2022;74:e1-20. https://doi.org/10.1002/art.42062.
Wang J, Chen H, Shi H, Zhang X, Shao Y, Hang B, et al. Effect of different doses of aspirin on the prognosis of Kawasaki disease. Pediatr Rheumatol. 2020;18:1–7. https://doi.org/10.1186/s12969-020-00432-x.
Sharathkumar AA, Faustino EVS, Takemoto CM. How we approach thrombosis risk in children with COVID-19 infection and MIS-C. Pediatr Blood Cancer. 2021;68:1–9. https://doi.org/10.1002/pbc.29049.
Al-Ghafry M, Vagrecha A, Malik M, Levine C, Uster E, Aygun B, et al. Multisystem inflammatory syndrome in children (MIS-C) and the prothrombotic state: Coagulation profiles and rotational thromboelastometry in a MIS-C cohort. J Thromb Haemost. 2021;19:1764–70. https://doi.org/10.1111/jth.15340.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Hadi Sahrai, Mahdi Hemmati-Ghavshough, Marzieh Shahrabi, Amir Hossein Jafari-Rouhi and Mohammad Solduzian declare that they have no conflicts of interest.
Authors contributions
Main idea, review, editing and supervision: Dr Mohammad Solduzian; literature search, data collection and writing first draft of the manuscript: Hadi Sahrai, Mahdi Hemmati-Ghavshough and Marzieh Shahrabi; Supervision: Dr Amir Hossein Jafari-Rouhi.
Data availability statement
Data sharing is not applicable to this manuscript as no datasets were generated during the writing of the review article.
Ethic approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahrai, H., Hemmati-Ghavshough, M., Shahrabi, M. et al. Thromboprophylaxis for Coagulopathy Related to COVID-19 in Pediatrics: A Narrative Review. Pediatr Drugs 25, 443–452 (2023). https://doi.org/10.1007/s40272-023-00566-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00566-x