Abstract
Background
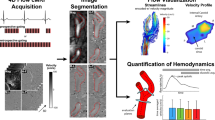
Three-dimensional, ECG-gated, time-resolved, three-directional, velocity-encoded phase-contrast MRI (4D flow MRI) has been applied extensively to measure blood velocity in great vessels but has been much less used in diseased carotid arteries. Carotid artery webs (CaW) are non-inflammatory intraluminal shelf-like projections into the internal carotid artery (ICA) bulb that are associated with complex flow and cryptogenic stroke.
Purpose
Optimize 4D flow MRI for measuring the velocity field of complex flow in the carotid artery bifurcation model that contains a CaW.
Methods
A 3D printed phantom model created from computed tomography angiography (CTA) of a subject with CaW was placed in a pulsatile flow loop within the MRI scanner. 4D Flow MRI images of the phantom were acquired with five different spatial resolutions (0.50–2.00 mm3) and four different temporal resolutions (23–96 ms) and compared to a computational fluid dynamics (CFD) solution of the flow field as a reference. We examined four planes perpendicular to the vessel centerline, one in the common carotid artery (CCA) and three in the internal carotid artery (ICA) where complex flow was expected. At these four planes pixel-by-pixel velocity values, flow, and time average wall shear stress (TAWSS) were compared between 4D flow MRI and CFD.
Hypothesis
An optimized 4D flow MRI protocol will provide a good correlation with CFD velocity and TAWSS values in areas of complex flow within a clinically feasible scan time (~ 10 min).
Results
Spatial resolution affected the velocity values, time average flow, and TAWSS measurements. Qualitatively, a spatial resolution of 0.50 mm3 resulted in higher noise, while a lower spatial resolution of 1.50–2.00 mm3 did not adequately resolve the velocity profile. Isotropic spatial resolutions of 0.50–1.00 mm3 showed no significant difference in total flow compared to CFD. Pixel-by-pixel velocity correlation coefficients between 4D flow MRI and CFD were > 0.75 for 0.50–1.00 mm3 but were < 0.5 for 1.50 and 2.00 mm3. Regional TAWSS values determined from 4D flow MRI were generally lower than CFD and decreased at lower spatial resolutions (larger pixel sizes). TAWSS differences between 4D flow and CFD were not statistically significant at spatial resolutions of 0.50–1.00 mm3 but were different at 1.50 and 2.00 mm3. Differences in temporal resolution only affected the flow values when temporal resolution was > 48.4 ms; temporal resolution did not affect TAWSS values.
Conclusion
A spatial resolution of 0.74–1.00 mm3 and a temporal resolution of 23–48 ms (1–2 k-space segments) provides a 4D flow MRI protocol capable of imaging velocity and TAWSS in regions of complex flow within the carotid bifurcation at a clinically acceptable scan time.







Similar content being viewed by others
References
Nayak, K. S., J.-F. Nielsen, M. A. Bernstein, et al. Cardiovascular magnetic resonance phase contrast imaging. J. Cardiovasc. Magn. Reson. 17(1):71, 2015. https://doi.org/10.1186/s12968-015-0172-7.
Markl, M., A. Frydrychowicz, S. Kozerke, M. Hope, and O. Wieben. 4D flow MRI. J. Magn. Reson. Imaging. 36(5):1015–1036, 2012. https://doi.org/10.1002/jmri.23632.
Szajer, J., and K. Ho-Shon. A comparison of 4D flow MRI-derived wall shear stress with computational fluid dynamics methods for intracranial aneurysms and carotid bifurcations—a review. J. Magn. Reson. Imaging. 48:62–69, 2018. https://doi.org/10.1016/j.mri.2017.12.005.
Chatzimavroudis, G. P., J. N. Oshinski, R. H. Franch, et al. Evaluation of the precision of magnetic resonance phase velocity mapping for blood flow measurements. J. Cardiovasc. Magn. Reson. 3(1):11–19, 2001. https://doi.org/10.1081/JCMR-100000142.
Nilsson, A., K. M. Bloch, J. Töger, E. Heiberg, and F. Ståhlberg. Accuracy of four-dimensional phase-contrast velocity mapping for blood flow visualizations: a phantom study. Acta Radiol. 54(6):663–671, 2013. https://doi.org/10.1177/0284185113478005.
Montalba, C., J. Urbina, J. Sotelo, et al. Variability of 4D flow parameters when subjected to changes in MRI acquisition parameters using a realistic thoracic aortic phantom. Magn. Reson. Med. 79(4):1882–1892, 2018. https://doi.org/10.1002/mrm.26834.
Zimmermann, J., D. Demedts, H. Mirzaee, et al. Wall shear stress estimation in the aorta: Impact of wall motion, spatiotemporal resolution, and phase noise. J. Magn. Reson. Imaging. 2018. https://doi.org/10.1002/jmri.26007.
Shen, X., S. Schnell, A. J. Barker, et al. Voxel-by-voxel 4D flow MRI-based assessment of regional reverse flow in the aorta. J. Magn. Reson. Imaging. 47(5):1276–1286, 2018. https://doi.org/10.1002/jmri.25862.
Callaghan, F. M., R. Kozor, A. G. Sherrah, et al. Use of multi-velocity encoding 4D flow MRI to improve quantification of flow patterns in the aorta. J. Magn. Reson. Imaging. 43(2):352–363, 2016. https://doi.org/10.1002/jmri.24991.
Garcia, J., A. J. Barker, and M. Markl. The role of imaging of flow patterns by 4D flow MRI in aortic stenosis. JACC Cardiovasc. Imaging. 12(2):252–266, 2019. https://doi.org/10.1016/j.jcmg.2018.10.034.
Puiseux, T., A. Sewonu, O. Meyrignac, et al. Reconciling PC-MRI and CFD: an in-vitro study. NMR Biomed. 32(5):e4063, 2019. https://doi.org/10.1002/nbm.4063.
Kweon, J., D. H. Yang, G. B. Kim, et al. Four-dimensional flow MRI for evaluation of post-stenotic turbulent flow in a phantom: comparison with flowmeter and computational fluid dynamics. Eur. Radiol. 26(10):3588–3597, 2016. https://doi.org/10.1007/s00330-015-4181-6.
Ngo, M. T., C. I. Kim, J. Jung, et al. Four-dimensional flow magnetic resonance imaging for assessment of velocity magnitudes and flow patterns in the human carotid artery bifurcation: comparison with computational fluid dynamics. Diagnostics. 9(4):223, 2019. https://doi.org/10.3390/diagnostics9040223.
Ngo, M. T., U. Y. Lee, H. Ha, et al. Improving blood flow visualization of recirculation regions at carotid bulb in 4D flow MRI using semi-automatic segmentation with ITK-SNAP. Diagnostics. 11(10):1890, 2021. https://doi.org/10.3390/diagnostics11101890.
Roldán-Alzate, A., S. García-Rodríguez, P. V. Anagnostopoulos, et al. Hemodynamic study of TCPC using in vivo and in vitro 4D flow MRI and numerical simulation. J. Biomech. 48(7):1325–1330, 2015. https://doi.org/10.1016/j.jbiomech.2015.03.009.
Edelstein, W. A., M. Mahesh, and J. A. Carrino. MRI: time is dose–and money and versatility. J. Am. Coll. Radiol. 7(8):650–652, 2010. https://doi.org/10.1016/j.jacr.2010.05.002.
Sajed, P. I., J. N. Gonzalez, C. A. Cronin, et al. Carotid bulb webs as a cause of “cryptogenic” ischemic stroke. AJNR Am. J. Neuroradiol. 38(7):1399–1404, 2017. https://doi.org/10.3174/ajnr.A5208.
Haussen, D. C., J. A. Grossberg, S. Koch, et al. Multicenter experience with stenting for symptomatic carotid web. Intervent. Neurol. 2018. https://doi.org/10.1159/000489710.
Haussen, D. C., J. A. Grossberg, M. Bouslama, et al. Carotid web (intimal fibromuscular dysplasia) has high stroke recurrence risk and is amenable to stenting. Stroke. 48(11):3134–3137, 2017. https://doi.org/10.1161/strokeaha.117.019020.
Park, C. C., R. El Sayed, B. B. Risk, et al. Carotid webs produce greater hemodynamic disturbances than atherosclerotic disease: a DSA time–density curve study. J. Neurointerv. Surg. 2021. https://doi.org/10.1136/neurintsurg-2021-017588.
Ozaki, D., T. Endo, H. Suzuki, et al. Carotid web leads to new thrombus formation: computational fluid dynamic analysis coupled with histological evidence. Acta Neurochir. (Wien). 162(10):2583–2588, 2020. https://doi.org/10.1007/s00701-020-04272-2.
Antonowicz, A., K. Wojtas, Ł Makowski, W. Orciuch, and M. Kozłowski. Particle image velocimetry of 3D-printed anatomical blood vascular models affected by atherosclerosis. Materials. 16(3):1055, 2023.
Ford, M. D., H. N. Nikolov, J. S. Milner, et al. PIV-measured versus CFD-predicted flow dynamics in anatomically realistic cerebral aneurysm models. J. Biomech. Eng. 2008. https://doi.org/10.1115/1.2900724.
Raschi, M., F. Mut, G. Byrne, et al. CFD and PIV analysis of hemodynamics in a growing intracranial aneurysm. Int. J. Numer. Methods Biomed. Eng. 28(2):214–228, 2012. https://doi.org/10.1002/cnm.1459.
Mitsouras, D., T. C. Lee, P. Liacouras, et al. Three-dimensional printing of MRI-visible phantoms and MR image-guided therapy simulation. Magn. Reson. Med. 77(2):613–622, 2017. https://doi.org/10.1002/mrm.26136.
Object30Pro, Objet30 Pro Key Features: Create parts with the precision, look and feel of real production parts. https://www.javelin-tech.com/3d/stratasys-3d-printer/objet30-pro/.
Summers, P. E., D. W. Holdsworth, H. N. Nikolov, B. K. Rutt, and M. Drangova. Multisite trial of MR flow measurement: phantom and protocol design. J. Magn. Reson. Imaging. 21(5):620–631, 2005. https://doi.org/10.1002/jmri.20311.
Wilson, J. S., M. Islam, and J. N. Oshinski. In vitro validation of regional circumferential strain assessment in a phantom aortic model using cine displacement encoding with stimulated echoes MRI. J. Magn. Reson. Imaging. 55(6):1773–1784, 2022. https://doi.org/10.1002/jmri.27972.
Wu, S. P., S. Ringgaard, and E. M. Pedersen. Three-dimensional phase contrast velocity mapping acquisition improves wall shear stress estimation in vivo. J. Magn. Reson. Imaging. 22(3):345–351, 2004. https://doi.org/10.1016/j.mri.2004.01.002.
Markl, M., A. Harloff, T. A. Bley, et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J. Magn. Reson. Imaging. 25(4):824–831, 2007. https://doi.org/10.1002/jmri.20871.
Stalder, A. F., M. F. Russe, A. Frydrychowicz, et al. Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magn. Reson. Med. 60(5):1218–1231, 2008. https://doi.org/10.1002/mrm.21778.
Ku, D. N., and D. P. Giddens. Laser Doppler anemometer measurements of pulsatile flow in a model carotid bifurcation. J. Biomech. 20:407–421, 1987. https://doi.org/10.1016/0021-9290(87)90048-0.
Ku, D. N., D. P. Giddens, D. J. Phillips, and D. E. Strandness. Hemodynamics of the normal human carotid bifurcation: in vitro and in vivo studies. Ultrasound. Med. Biol. 11(1):13–26, 1985. https://doi.org/10.1016/0301-5629(85)90003-1.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 5(3):293–302, 1985. https://doi.org/10.1161/01.ATV.5.3.293.
Markl, M., F. Wegent, T. Zech, et al. In vivo wall shear stress distribution in the carotid artery. Circ. Cardiovasc. Imaging. 3(6):647–655, 2010. https://doi.org/10.1161/CIRCIMAGING.110.958504.
Frydrychowicz, A., A. Berger, M. F. Russe, et al. Time-resolved magnetic resonance angiography and flow-sensitive 4-dimensional magnetic resonance imaging at 3 Tesla for blood flow and wall shear stress analysis. J. Thoracic. Cardiovasc. Surg. 136(2):400–407, 2008. https://doi.org/10.1016/j.jtcvs.2008.02.062.
Frydrychowicz, A., A. F. Stalder, M. F. Russe, et al. Three-dimensional analysis of segmental wall shear stress in the aorta by flow-sensitive four-dimensional-MRI. J. Magn. Reson. Imaging. 30(1):77–84, 2009. https://doi.org/10.1002/jmri.21790.
Harloff, A., T. Zech, F. Wegent, et al. Comparison of blood flow velocity quantification by 4D flow MR imaging with ultrasound at the carotid bifurcation. AJNR Am. J. Neuroradiol. 34(7):1407–1413, 2013. https://doi.org/10.3174/ajnr.A3419.
Medero, R., C. Hoffman, and A. Roldán-Alzate. Comparison of 4D flow MRI and particle image velocimetry using an in vitro carotid bifurcation model. Ann. Biomed. Eng. 46(12):2112–2122, 2018. https://doi.org/10.1007/s10439-018-02109-9.
Cibis, M., W. V. Potters, F. J. Gijsen, et al. The effect of spatial and temporal resolution of cine phase contrast MRI on wall shear stress and oscillatory shear index assessment. PLoS One. 11(9):e0163316–e0163316, 2016. https://doi.org/10.1371/journal.pone.0163316.
Oktar, S. O., C. Yücel, D. Karaosmanoglu, et al. Blood-flow volume quantification in internal carotid and vertebral arteries: comparison of 3 different ultrasound techniques with phase-contrast MR imaging. AJNR Am. J. Neuroradiol. 27(2):363–369, 2006.
Potters, W. V., H. A. Marquering, E. VanBavel, and A. J. Nederveen. Measuring wall shear stress using velocity-encoded MRI. Curr. Cardiovasc. Imaging Rep. 7(4):9257, 2014. https://doi.org/10.1007/s12410-014-9257-1.
Petersson, S., P. Dyverfeldt, and T. Ebbers. Assessment of the accuracy of MRI wall shear stress estimation using numerical simulations. J. Magn. Reson. Imaging. 36(1):128–138, 2012. https://doi.org/10.1002/jmri.23610.
Potters, W.V., P. van Ooij, H. Marquering, E. vanBavel, and A.J. Nederveen, Volumetric arterial wall shear stress calculation based on cine phase contrast MRI. J. Magn. Reson. Imaging 2015 41(2):505–516. https://doi.org/10.1002/jmri.24560.
Bae, T., J. H. Ko, and J. Chung. Turbulence intensity as an indicator for ischemic stroke in the carotid web. World Neurosurg. 2021. https://doi.org/10.1016/j.wneu.2021.07.049.
Choi, P. M. C., D. Singh, A. Trivedi, et al. Carotid webs and recurrent ischemic strokes in the era of CT angiography. AJNR Am. J. Neuroradiol. 36(11):2134–2139, 2015. https://doi.org/10.3174/ajnr.A4431.
Compagne, K. C. J., K. Dilba, E. J. Postema, et al. Flow patterns in carotid webs: a patient-based computational fluid dynamics study. AJNR Am. J. Neuroradiol. 40(4):703–708, 2019. https://doi.org/10.3174/ajnr.A6012.
Kumar, D. R., E. Hanlin, I. Glurich, J. J. Mazza, and S. H. Yale. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin. Med. Res. 8(3–4):168–172, 2010. https://doi.org/10.3121/cmr.2009.866.
Lee, B. K. Computational fluid dynamics in cardiovascular disease. Korean Circ. J. 41(8):423–430, 2011. https://doi.org/10.4070/kcj.2011.41.8.423.
Iffrig, E., L. H. Timmins, R. El Sayed, W. R. Taylor, and J. N. Oshinski. A new method for quantifying abdominal aortic wall shear stress using phase contrast magnetic resonance imaging and the Womersley solution. J. Biomech. Eng. 2022. https://doi.org/10.1115/1.4054236.
Katritsis, D., L. Kaiktsis, A. Chaniotis, et al. Wall shear stress: theoretical considerations and methods of measurement. Prog. Cardiovasc. Dis. 49(5):307–329, 2007. https://doi.org/10.1016/j.pcad.2006.11.001.
Acknowledgements
The authors would like to acknowledge Paul Lee, Ph.D. at Emory University for the help in operating Object 30 3D printer.
Funding
This work was funded by the American Heart Association Grant Nos. 0000065426 (Sharifi) and 19IPLOI34760670 (Allen), National Institutes of Health Grant Nos. R21NS114603 (Allen and Oshinski) and R01EB027774 (Oshinski). Additionally, this material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1937971 (El Sayed). Any opinions, findings, and conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict or financial interests in this manuscript.
Human, Animal, and Informed Consent
No human or animal studies were carried out by the authors of this article.
Additional information
Associate Editor Keefe B. Manning oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Sayed, R., Sharifi, A., Park, C.C. et al. Optimization of 4D Flow MRI Spatial and Temporal Resolution for Examining Complex Hemodynamics in the Carotid Artery Bifurcation. Cardiovasc Eng Tech 14, 476–488 (2023). https://doi.org/10.1007/s13239-023-00667-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-023-00667-1