Abstract
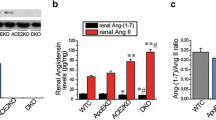
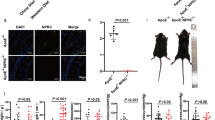
As the most common cardiovascular disease, atherosclerosis (AS), is a leading cause of high mortality in patients with chronic renal failure. Rab27a has been reported to regulate the progression of cardiovascular and renal diseases. Nevertheless, little studies investigated the role and mechanism of Rab27a in uremic-accelerated AS (UAAS). An animal model of UAAS was established in apolipoprotein E knockout (apoE−/−) mice using 5/6 nephrectomy (NX). We conducted in vitro and in vivo functional experiments to explore the role of Rab27a in UAAS, including the presence of oxidized low-density lipoprotein (ox-LDL). Rab27a expression was upregulated in the plaque tissues of NX apoE−/− mice. The knockout of Rab27a (Rab27a−/−) reduced AS-induced artery injury, as manifested by the reductions of plaque area, collagen deposition, inflammation and lipid droplet. Besides, cholesterol efflux was increased, while the expression of lipid metabolism-related proteins and the secretions of pro-inflammatory factors were decreased in ox-LDL-induced NX Rab27a−/− apoE−/− mice group. Further, Rab27a deletion inhibited the activation of nuclear factor κB (NF-κB) pathway. In conclusion, our study indicated that Rab27a deficiency attenuated foam cell formation and macrophage inflammation, depending on the NF-κB pathway activation, to inhibit AS progression in uremic apoE−/− mice. This finding may provide a new targeting strategy for UAAS therapy.




Similar content being viewed by others
Data Availability
The data set used and/or analyzed in the current study can be obtained from the corresponding author upon reasonable request.
Change history
14 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10735-023-10138-5
References
An HJ, Song DH, Koh HM, Ko GH, Lee JH, Kim DC, Yang JW, Kim MH, Seo DH, Jang SM, Lee JS (2019) RAB27A is an independent prognostic factor in clear cell renal cell carcinoma. Biomark Med 13(4):239–247
Andrés-Manzano MJ, Andrés V, Dorado B (2015) Oil red O and hematoxylin and eosin staining for quantification of atherosclerosis burden in mouse aorta and aortic root. Methods Mol Biol 1339(6):85–99
Aono J, Suzuki J, Iwai M, Horiuchi M, Nagai T, Nishimura K, Inoue K, Ogimoto A, Okayama H, Higaki J (2012) Deletion of the angiotensin II type 1a receptor prevents atherosclerotic plaque rupture in apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol 32(6):1453–1459
Bhuin T, Roy JK (2009) Rab11 is required for embryonic nervous system development in Drosophila. Cell Tissue Res 335(2):349–356
Bisgaard LS, Bosteen MH, Fink LN, Sorensen CM, Rosendahl A, Mogensen CK, Rasmussen SE, Rolin B, Nielsen LB, Pedersen TX (2016) Liraglutide reduces both atherosclerosis and kidney inflammation in moderately uremic LDLr−/− mice. PLoS ONE 11(12):e0168396
Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN (2017) Mechanisms of foam cell formation in atherosclerosis. J Mol Med 95(11):1153–1165
Dai Y, Wu X, Dai D, Li J, Mehta JL (2018) MicroRNA-98 regulates foam cell formation and lipid accumulation through repression of LOX-1. Redox Biol 16:255–262
Ding X, Jing N, Shen A, Guo F, Zhao Y (2020) MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J Endocrinol Invest 44:1175
Feng SJ, Li H, Wang SX (2015) Lower hydrogen sulfide is associated with cardiovascular mortality, which involves cPKCβII/Akt pathway in chronic hemodialysis patients. Blood Purif 40(3):260–269
Feng F, Jiang Y, Lu H, Lu X, Wang S, Wang L, Wei M, Lu W, Du Z, Ye Z, Yang G, Yuan F, Ma Y, Lei X, Lu Z (2016) Rab27A mediated by NF-κB promotes the stemness of colon cancer cells via up-regulation of cytokine secretion. Oncotarget 7(39):63342–63351
Feng Y, Zhong X, Tang TT, Wang C, Wang LT, Li ZL, Ni HF, Wang B, Wu M, Liu D, Liu H, Tang RN, Liu BC, Lv LL (2020) Rab27a dependent exosome releasing participated in albumin handling as a coordinated approach to lysosome in kidney disease. Cell Death Dis 11(7):513
Gerber PP, Cabrini M, Jancic C, Paoletti L, Banchio C, von Bilderling C, Sigaut L, Pietrasanta LI, Duette G, Freed EO, Basile Gde S, Moita CF, Moita LF, Amigorena S, Benaroch P, Geffner J, Ostrowski M (2015) Rab27a controls HIV-1 assembly by regulating plasma membrane levels of phosphatidylinositol 4,5-bisphosphate. J Cell Biol 209(3):435–452
Glaucylara GR, Peter L (2018) Atherosclerosis and inflammation: overview and updates. Clin Sci 132(12):1243–1252
Gross T, Wack G, Syhr KMJ, Tolmachova T, Seabra MC, Geisslinger G, Niederberger E, Schmidtko A, Kallenborn-Gerhardt W (2020) Rab27a contributes to the processing of inflammatory pain in mice. Cells 9(6):1488
Guo K, Hu L, Xi D, Zhao J, Liu J, Luo T, Ma Y, Lai W, Guo Z (2018) PSRC1 overexpression attenuates atherosclerosis progression in apoE -/ mice by modulating cholesterol transportation and inflammation. J Mol Cell Cardiol 116:69–80
Jordens I, Marsman M, Kuijl C, Neefjes J (2005) Rab proteins, connecting transport and vesicle fusion. Traffic 6(12):1070–1077
Kaseda R, Tsuchida Y, Yang H-C, Yancey PG, Zhong J, Tao H, Bian A, Fogo AB, Linton MRF, Fazio S (2018) Chronic kidney disease alters lipid trafficking and inflammatory responses in macrophages: effects of liver X receptor agonism. BMC Nephrol 19(1):17
Layoun A, Samba M, Santos MM (2015) Isolation of murine peritoneal macrophages to carry out gene expression analysis upon toll-like receptors stimulation. J Vis Exp. https://doi.org/10.3791/52749
Li ZG, Scott MJ, Brzóska T, Sundd P, Li YH, Billiar TR, Wilson MA, Wang P, Fan J (2018) Lung epithelial cell-derived IL-25 negatively regulates LPS-induced exosome release from macrophages. Military Med Res 5(1):24
Li S, Jia Y, Xue M, Hu F, Zheng Z, Zhang S, Ren S, Yang Y, Si Z, Wang L, Guan M, Xue Y (2020) Inhibiting Rab27a in renal tubular epithelial cells attenuates the inflammation of diabetic kidney disease through the miR-26a-5p/CHAC1/NF-kB pathway. Life Sci 261:118347
Libby P, Ridker PM, Hansson GK (2012) Inflammation in atherosclerosis. J Assoc Physicians India 32(9):2045–2051
Lightbody RJ, Taylor JMW, Dempsie Y, Graham A (2020) MicroRNA sequences modulating inflammation and lipid accumulation in macrophage “foam” cells: implications for atherosclerosis. World J Cardiol 12(7):303–333
Liu J, Gong X, Zhu X, Xue D, Liu Y, Wang P (2017a) Rab27A overexpression promotes bladder cancer proliferation and chemoresistance through regulation of NF-κB signaling. Oncotarget 8(43):75272–75283
Liu Z, Zhu H, Dai X, Wang C, Zou MH (2017b) Macrophage liver kinase B1 inhibits foam cell formation and atherosclerosis. Circ Res 121(9):1047. https://doi.org/10.1161/CIRCRESAHA.117.311546
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Luo Y, Yu MH, Yan YR, Zhou Y, Qin SL, Huang YZ, Qin J, Zhong M (2020) Rab27A promotes cellular apoptosis and ROS production by regulating the miRNA-124-3p/STAT3/RelA signalling pathway in ulcerative colitis. J Cell Mol Med 19:11330
Martinez-Arroyo O, Ortega A, Perez-Hernandez J, Chaves FJ, Cortes R (2020) Rab-rabphilin system in injured human podocytes stressed by glucose overload and angiotensin II. Am J Physiol Renal Physiol 319(2):F178
Nyandwi JB, Ko YS, Jin H, Yun SP, Park SW, Kim HJ (2021) Rosmarinic acid increases macrophage cholesterol efflux through regulation of ABCA1 and ABCG1 in different mechanisms. Int J Mol Sci 22(16):8791
Patel KM, Strong A, Tohyama J, Jin X, Morales CR, Billheimer J, Millar J, Kruth H, Rader DJ (2015) Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res 116(5):789–796
Plotkin JD, Elias MG, Dellinger AL, Kepley CL (2017) NF-κB inhibitors that prevent foam cell formation and atherosclerotic plaque accumulation. Nanomed Nanotechnol Biol Med 13(6):2037–2048
Schwarz A, Bonaterra GA, Schwarzbach H, Kinscherf RJJoBS (2017) Oxidized LDL-induced JAB1 influences NF-κB independent inflammatory signaling in human macrophages during foam cell formation. J Biomed Sci 24(1):12
Scott RP, Quaggin SE (2015) The cell biology of renal filtration. J Cell Biol 209(2):199–210
Shen Y, Yuan Z, Yin A, Liu Y, Xiao Y, Wu Y, Wang L, Liang X, Zhao Y, Tian Y (2011) Antiatherogenic effect of pioglitazone on uremic apolipoprotein E knockout mice by modulation of the balance of regulatory and effector T cells. Atherosclerosis 218(2):330–338
Shi H, Zhang C, Pasupuleti V, Hu X, Prosdocimo DA, Wu W, Qing Y, Wu S, Mohammad H, Gerson SL (2017) CCN3 regulates macrophage foam cell formation and atherosclerosis. Am J Pathol 187(6):1230–1237
Shimada-Sugawara M, Sakai E, Okamoto K, Fukuda M, Izumi T, Yoshida N, Tsukuba T (2015) Rab27A regulates transport of cell surface receptors modulating multinucleation and lysosome-related organelles in osteoclasts. Sci Rep 5:1
Verma K, Srivastava VK, Datta S (2020) Rab GTPases take centre stage in understanding Entamoeba histolytica biology. Small GTPases 11(5):320–333
Wan X, Chen X, Liu L, Zhao Y, Huang W-J, Zhang Q, Miao G-G, Chen W, Xie H-G, Cao C-C (2013) Berberine ameliorates chronic kidney injury caused by atherosclerotic renovascular disease through the suppression of NFκB signaling pathway in rats. PLoS ONE 8(3):e59794
Wang Q, Ni Q, Wang X, Zhu H, Wang Z, Huang JJMO (2015) High expression of RAB27A and TP53 in pancreatic cancer predicts poor survival. Med Oncol 32(1):372
Wang C, Yang W, Liang X, Song W, Lin J, Sun Y, Guan X (2020) MicroRNA-761 modulates foam cell formation and inflammation through autophagy in the progression of atherosclerosis. Mol Cell Biochem 475:135
Wayne EC, Long C, Haney MJ, Batrakova EV, Kabanov AV (2019) Targeted delivery of siRNA lipoplexes to cancer cells using macrophage transient horizontal gene transfer. Adv Sci 6:19
Xiong R, Lu X, Song J, Li H, Wang S (2019a) Molecular mechanisms of hydrogen sulfide against uremic accelerated atherosclerosis through cPKCβII/Akt signal pathway. BMC Nephrol 20(1):358
Xiong R, Lu X, Song J, Li H, Wang SJBN (2019b) Molecular mechanisms of hydrogen sulfide against uremic accelerated atherosclerosis through cPKCβII/Akt signal pathway. BMC Nephrol 20(1):1–7
Yu X-H, Zheng X-L, Tang C-K (2015) Nuclear factor-κB activation as a pathological mechanism of lipid metabolism and atherosclerosis. Adv Clin Chem 70:1–30
Yu F, Wu W, Liang M, Huang Y, Chen C (2020) Prognostic significance of Rab27A and Rab27B expression in esophageal squamous cell cancer. Cancer Manag Res 12:6353–6361
Zhang C, Qin J-J, Gong F-H, Tong J-J, Cheng W-L, Wang H, Zhang Y, Zhu X, She Z-G, Xia H (2018) Mindin deficiency in macrophages protects against foam cell formation and atherosclerosis by targeting LXR-β. Clin Sci 132(11):1199–1213
Zhong Y, Liu C, Feng J, Li JF, Fan ZC (2020) Curcumin affects ox-LDL-induced IL-6, TNF-α, MCP-1 secretion and cholesterol efflux in THP-1 cells by suppressing the TLR4/NF-κB/miR33a signaling pathway. Exp Ther Med 20(3):1856–1870
Zhou M, Ren P, Zhang Y, Li S, Li M, Li P, Shang J, Liu W, Liu H (2019) Shen-Yuan-Dan capsule attenuates atherosclerosis and foam cell formation by enhancing autophagy and inhibiting PI3K/Akt/mTORC1 signaling pathway. Front Pharmacol 10:603
Zuo Y, Yancey P, Castro I, Khan W, Motojima M, Ichikawa I, Fogo AB, Linton MF, Fazio S, Kon V (2009) Renal dysfunction potentiates foam cell formation by repressing ABCA1. Arterioscler Thromb Vasc Biol 29(9):1277–1282
Acknowledgements
Not Applicable.
Funding
This research was supported by National Natural Science Foundation of China (Grant No. 81200541), ‘PRO•Run’ Fund of the Nephrology Group of CEBM (Grant No. KYS2021-03-02-9), Key Research and Development Program of Shaanxi (Grant No. 2020KW-043), Xi’an Science and Technology Project (Grant No. 201805095YX3SF29), The Fundamental Research Funds for the Central Universities (Grant No. xjj2017047), Shaanxi Provincial Health and Family Planning Scientific Research Fund Project, China (Grant No. 2016D064) and China Scholarship Fund (Grant No. 201506285033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they had no competing interests to the research, authorship, and/or publication of this article.
Ethical approval
The study was approved by the institutional ethics committee for animal experiments of Xi’an Jiaotong University (No. 2012-600). And carried out in accordance with the guidelines of Laboratory Animal Management Regulations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, Y., Gao, Y., Fu, J. et al. Lack of Rab27a attenuates foam cell formation and macrophage inflammation in uremic apolipoprotein E knockout mice. J Mol Histol 54, 183–193 (2023). https://doi.org/10.1007/s10735-023-10125-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-023-10125-w