Abstract
Bacteroides fragilis is an important etiological agent of serious infections in humans. Rapid methods, readily adaptable to use in medical laboratories, are needed to detect antibiotic resistance and decrease the likelihood of therapy failure. The aim of this study was to determine the prevalence of B. fragilis cfiA-positive isolates. The second purpose was to investigate the carbapenemase activity in B. fragilis strains by Carba NP test. In the study, 5.2% of B. fragilis isolates are phenotypically resistant to meropenem. The cfiA gene was identified in 6.1% of B. fragilis isolates. The MICs of meropenem were significantly higher in cfiA-positive strains. The presence of the cfiA gene along with the IS1186 was detected in one B. fragilis strain which was resistant to meropenem (MIC 1.5 mg/L). The Carba NP test results were positive for all the cfiA-positive strains, including those susceptible to carbapenems based on their MIC values. A review of the literature revealed that the rate of B. fragilis with the cfiA gene varies from 7.6 to 38.9% worldwide. Presented results are in line with the other European studies. Phenotypic testing with the Carba NP test, it seems to be a viable alternative for the cfiA gene detection in B. fragilis isolates. The positive result obtained is of greater clinical importance than the detection of the gene cfiA.
Similar content being viewed by others
Introduction
Members of the genus Bacteroides are a component of the human microbiota. They colonize the gastrointestinal tract, distal part of the genitourinary system, and the upper airways. Many species are opportunistic pathogens, responsible for endogenous infections [1, 2, 3].
Bacteroides fragilis is considered the most important species, with infection rates of 60–80% and is the most frequently identified anaerobic bacteria (excluding Clostridioides difficile recovered from patients with antibiotic diarrhea) in the clinical laboratories. It is isolated from mono- and polymicrobial infections. It occurs in specimens taken from sites of infection following violation of natural barriers by surgery, inflammation, or trauma. Intra-abdominal infections are the most common form of infection [1, 4].
Laboratory diagnostics of anaerobes is one of the most challenging aspects of clinical bacteriology. Bacteroides sp. isolation, identification, and antibiotic susceptibility testing (AST) is time consuming and labor intensive. Thus, anaerobes can be omitted from the routine diagnostic in many medical laboratories [5, 6, 7, 8, 9].
Infections caused by B. fragilis should be treated according to the results of AST because of an increasing resistance to commonly used antibiotics including β-lactams, tetracyclines, macrolides, and fluoroquinolones [8]. Carbapenems are among the most effective drugs and are considered the drug of choice for therapy of complicated intra-abdominal infections, acute gynecological infections, and skin and soft tissue infections caused by B. fragilis. Such infections are often polybacterial and also caused by other Gram-negative bacilli [10, 11].
Clinical isolates of B. fragilis may be resistant to carbapenems, considered to be the last chance β-lactam antibiotic, so resistance should be monitored extensively [12, 13, 14, 15, 16].
Resistance to carbapenems in B. fragilis is usually caused by the expression of the class B metallo-beta-lactamase encoded by, located on the chromosome cfiA gene. cfiA encodes an Ambler class B zinc metallo-β-lactamase (MBL) that can hydrolyzes most of β-lactams, including cephamycins and carbapenems [10, 17]. On this basis, Bacteroides spp. can be classified into Division I (cfiA-negative) and Division II (cfiA-positive) [17]. Anaerobes that produce MBL enzyme are the most worrisome. They hydrolyze nearly all β-lactam antibiotics, except monobactam [10, 18, 19]. These β-lactamase are not inactivated by currently known β-lactamase inhibitors [18]. cfiA gene is considered as a silent gene with a low level of constitutive expression. Its expression can be upregulated following the insertion of an insertion sequence (IS) with an efficient promotor immediately upstream of the gene [17, 20].
Rapid methods, readily adaptable and optimized for use in medical laboratories, are needed to detect antibiotic resistance in anaerobic bacteria and decrease the likelihood of carbapenem therapy failure [21].
Detection of cfiA gene is not an optimal method for routine identification of strains resistant to carbapenems. Proteins cannot be expressed at a sufficiently high level to classify the strains as resistant [18, 22]. Phenotypic imipenem-EDTA double disk synergy test for the detection of metallo‐β‐lactamases produced by Gram-negative aerobic bacilli, has no or restricted application with anaerobic bacteria. B. fragilis isolates with the cfiA gene can be susceptible or intermediate to imipenem and have a negative imipenem double-ended E-test result but be resistant to meropenem [23, 24]. Double-ended E-test strips impregnated with meropenem or imipenem with or without EDTA has been proposed as well. A preliminary analysis indicated that sensitivity is highly variable and depends on the carbapenem used and the resistance level of the strains tested [21, 24].
A potentially applicable for routine use method identifying carbapenemase production is a biochemical method relying on imipenem hydrolysis—the Carba NP test, originally intended for the aerobic Enterobacteriaceae bacilli [25, 26]. There are several reports concerning the application of Carba NP test for anaerobic bacteria [27, 28, 29]. The potential use of this method should be based on evidence resulting from studies with clinical strains performed under conditions simulating routine work in a clinical microbiology laboratory.
Aim
The aim of this study was to determine the prevalence of B. fragilis cfiA-positive (Division II) isolates to assess its influence on phenotypic resistance to carbapenems. The second purpose was to investigate the carbapenemase activity in B. fragilis strains by Carba NP test and to compare the outcome with the phenotypic (MICs evaluation) and genotypic (cfiA and IS genes detection) test results.
Materials and methods
Bacterial strains
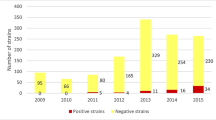
The study was performed at the microbiology laboratory that served bacteriological samples from a major academic hospital in Warsaw, Poland, the Medical University of Warsaw. Altogether, 115 consecutive non-duplicate B. fragilis isolates were analyzed over a period of 5 years between January 2013 and December 2017. Strains were cultured from the following clinical specimens: wound/abscess swabs (81), peritoneal cavity fluid (14), blood (6), soft tissue (5), others (9).
Clinical sample was plated on Schaedler agar media with 5% sheep blood, vitamin K, and hemin (bioMérieux, France) and was incubated at 37 °C in an anaerobic atmosphere (anaerostat Genbox System providing air composition: 85% N2, 10% H2, and 5% CO2; bioMérieux). Incubation period lasted 24–48 h. Bacterial identification was carried out using the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS, bioMérieux). All the strains were stored deep-frozen, in a temperature of − 70 °C, in bead vials Protect Select (Technical Service Consultants Ltd, UK). To perform phenotypic and molecular tests, the strains were revived by culturing on Schaedler agar.
Antibiotic susceptibility test and interpretation
The E-test strips impregnated with a concentration gradient of imipenem (0.002–32 mg/L) and meropenem (0.002–32 mg/L) were used for detection of a minimum inhibitory concentration (MIC) of carbapenems. E-test assays were performed as recommended by the manufacturer (bioMérieux, France). The interpretation was conducted in accordance with The European Committee on Antimicrobial Susceptibility Testing (EUCAST; version 12.0 which complies with version 13; year 2023) recommendations and according to results of Rennie et al. on the assessment of drug susceptibility of anaerobic bacteria [30, 31]. MIC90 and MIC50 values were defined as the lowest concentration of the antibiotic at which 90 and 50% of the isolates were inhibited, respectively. The strain from the American Type Culture Collection: Bacteroides fragilis ATCC 25285 was used as control. In 2022, EUCAST changed the interpretations of antibiotic susceptibility for Bacteroides spp. [30, 32]. The clinical MIC breakpoints for meropenem have been changed so that MIC breakpoint > 1 mg/L was interpreted as resistant to meropenem. According to the earlier version of EUCAST (v. 11.0 from 2021), a MIC of > 8 mg/L of meropenem indicated resistance to this antibiotic. Interpretation for imipenem has been withdrawn [30, 32].
The Carba NP test
The Carba NP (Carbapenemase Nordmann–Poirel) test is a phenotypic method that was developed to detect carbapenemase produced by Gram-negative aerobic bacteria, including Enterobacteriaceae and Pseudomonas aeruginosa isolates [33, 34]. A variant of the test, the CarbAcineto, allows for the detection of acquired carbapenemases in Acinetobacter spp. [25].
The Carba NP test is based on In vitro detection of hydrolysis of imipenem by a bacterial lysate suspended in a buffer containing phenol red. As a result of imipenem hydrolysis, the pH of the reaction medium decreases (acidification), which is observed as a change in the color of phenol red to yellow or orange. A positive result indicates carbapenemase production by the strain. Positive control (carbapenemase-producing isolate) and negative control (carbapenemase-not-producing isolate) were included in the study to assess the correctness of the test performed.
The Carba NP test was performed as follows: one loopful of bacteria, approximately 10 μL (incubated for 48 h at 30 ºC on Schaedler agar) was resuspended in a Tris–HCl 20 mmol/L lysis buffer (B-PERII, Bacterial Protein Extraction Reagent; Thermo Scientific Pierce, Rockford, IL, USA), vortexed for 1 min. and further incubated at room temperature for 30 min., then centrifuged at 10,000 × g at room temperature for 5 min. In the test tube, the supernatant was mixed with 100 µL of a 1 mL solution made of 3 mg of imipenem monohydrate (pH 7.8), phenol red solution (both Sigma, Saint-Quentin Fallavier, France) and 0.1 mmol/L ZnSO4 (Merck Millipore, Guyancourt, France). In the control tube, the supernatant was mixed with the phenol red solution (prepared by mixing 2 mL of a phenol red solution 0.5% (wt/vol) with 16.6 mL of distilled water). The pH value was then adjusted to using a pH meter to 7.8 by adding drops of 1 N NaOH.
A mixture was incubated at 37 °C for a maximum of 2 h. The test was read by comparing the color of the mixture in the test and the control tubes. When imipenem was hydrolyzed, the colur has been turned from red to orange or yellow, which was interpreted as a positive Carba NP test. Tubes containing bacterial extracts with no carbapenemase activity remained red (negative). In the case of a slight colour change, the result was considered invalid, and the test was repeated. A carbapenemase-producing strain BF8 B. fragilis (BFr81) was used as the positive control, and B. fragilis ATCC 25285 strain as the negative control [28, 34].
The cfiA-mediated carbapenem resistance gene and insertion sequence-encoding genes detection
cfiA gene was detected by polymerase chain reaction (PCR) using specific primer pairs [35]. The isolates identified as cfiA-positive were also evaluated for insertion sequence-encoding genes (IS1186, IS1187, IS1188, IS942) [36, 37]. The DNA was collected using a genomic DNA isolation kit for bacteria, cell cultures, and solid tissue (Genomic Mini; A&A Biotechnology, Poland). The starters were ordered and synthesized at the Laboratory of DNA Sequencing and Oligonucleotide Synthesis at the Institute of Biochemistry and Biophysics Polish Academy of Sciences (Warsaw, Poland). The obtained DNA fragments were subsequently separated using electrophoresis in 1% agarose gel with ethidium bromide to identify PCR products and then observed in a gel imaging device. BF8 B. fragilis (BFr81) was used as the positive control in the PCR test. PCR primers and conditions are listed in Table 1.
Statistical analysis
Statistical analysis was conducted with Statistica 10 (StatSoft, Inc.). Any correlations between the presence of resistant gene (cfiA) in the evaluated isolates and antibiotic MIC values were analyzed with linear regression using the Pearson method. The obtained correlation coefficients (r) were interpreted as follows: r = 0, no correlation; 0 < r ≤ 0.1, very weak correlation; 0.1 < r ≤ 0.3, weak correlation; 0.3 < r ≤ 0.5, moderate correlation; 0.5 < r ≤ 0.7, strong correlation; 0.7 < r ≤ 0.9, very strong correlation; 0.9 < r < 1, almost perfect correlation; and r = 1, perfect correlation. The p value was calculated for each correlation coefficient and was considered statistically significant at p ≤ 0.05.
Results
Using the currently applicable criteria for interpreting phenotypic antibiotic susceptibility tests according to the recommendations of EUCAST, it has been shown that 5.2% (6/115) of B. fragilis strains are resistant to meropenem. Assuming earlier (version 11.0; EUCAST, 2021) breakpoint values [32], only two (1.73%) strains resistant to meropenem and one (0.87%) intermediate could be identified in the tested pool of clinical strains (Table 2). According to the up-to-date recommendations, isolates with an MIC of 1 mg/L may harbor the cfiA gene. Table 2. Characterizes isolates that are phenotypically resistant to any of the carbapenems and/or had detected sequences that may be associated with drug resistance to these antibiotics. Imipenem MIC50 and MIC90 were 0.125 and 0.87 mg/L, respectively. Meropenem MIC50 and MIC90 were 0.094 and 0.25 mg/L, respectively.
The cfiA gene was identified in 7/115 B. fragilis isolates (6.1%). The meropenem resistance in B. fragilis isolates, calculated according to the breakpoint reported in the version 12 and 13 EUCAST guidelines, was significantly higher than the one calculated following the v. 11 EUCAST guidelines (75% vs. 25%, p < 0.05). In cfiA-positive isolates, the MIC values were significantly higher for meropenem than for imipenem which proves that the use of meropenem better identifies carbapenem resistance in phenotypic testing. The meropenem MIC for cfiA-positive strains ranged from 1 to 32 mg/L and for cfiA-negative from 0.002 to 1 mg/L. The results of antibiotic susceptibility testing of all tested strains are included in the supplementary material.
Figures 1 and 2 depict the correlation between imipenem and meropenem MIC values and the cfiA gene presence in B. fragilis isolates.
The presence of the cfiA gene weakly correlates with the MIC of imipenem and moderately correlates with the MIC of meropenem. The Pearson correlation coefficient equals 0.17 and 0.35 for imipenem and meropenem, respectively. The IS1186 sequence was detected in one strain. The presence of the cfiA gene along with the IS1186 was detected in a B. fragilis strain, which was susceptible to imipenem (MIC 0.125 mg/L) and resistant to meropenem (MIC 1.5 mg/L). No other insertion sequences (IS1187, IS1188, IS942) were detected in the screened strains.
The Carba NP test results were positive for all (seven) of the cfiA-positive strains, including two isolates susceptible to carbapenems based on their MIC values (0.5 and 1 mg/L). In two isolates that were phenotypically susceptible to meropenem (MIC 0.5 mg/L and 1 mg/L), cfiA gene and carbapenemase production were detected. In one B. fragilis strain (MIC of meropenem, 4 mg/L), the production of metallo-beta-lactamase was not detected (cfiA-negative), so other mechanisms produce carbapenem resistance.
Discussion
B. fragilis is of particular clinical significance because of its numerous virulence factors such as capsular polysaccharides, iron acquisition, survival during the prolonged oxidative stress, quorum sensing, secretion of extracellular and histolytic enzymes, type VI secretion systems, and natural or acquired resistance to multiple antibiotics [38]. The plasticity of the B. fragilis genome allows it to incorporate virulence and related to antibiotic resistance determinants via horizontal gene transfer and to switch specific resistance genes on or off [39]. B. fragilis is responsible for purulent-septic infections, causes infections that result in high mortality rate, especially in the case of bacteremia [2, 11]. Enterotoxigenic B. fragilis (ETBF) strains are strongly associated with the occurrence of inflammatory bowel disease, colitis-associated colorectal cancer as well [40, 41, 42].
The carbapenems, including imipenem and meropenem, are active against anaerobic bacteria, but due to carbapenem resistance becoming increasingly more widespread, their use should be reserved for serious infections. There are reports, indicating that the frequency of carbapenem-resistant strains isolation is on an upward trend. It is necessary to detect resistance in isolates from patients and to monitor this phenomenon using sensitive and simple tests that could be used in the routine work of medical laboratories.
The β-lactam antibiotics resistance among Bacteroides spp. results from differing mechanisms. Of the greatest clinical and epidemiological significance is the production of different classes of β-lactamases, including the CfiA carbapenemase which hydrolyzes penicillins, cephalosporins, and carbapenems [17].
Carbapenem resistance may have other genetic causes, such as or reduced permeability of the outer membrane, over-expression of efflux pump genes (role in multiresistance, promoting MDR in B. fragilis), or reduced affinity of penicillin binding proteins [1, 17, 39, 43].
This study determined the prevalence of B. fragilis cfiA-positive (Division II) isolates among strains isolated from infections of patients hospitalized in a large academic hospital in Warsaw, Poland. The influence of the presence of cfiA genes on phenotypic resistance to carbapenems was assessed, as well. The cfiA gene was identified in 6.1% B. fragilis isolates.
This subject has been widely studied by Jeverica et al. who screened a collection of B. fragilis isolates (623) using MALDI-TOF MS. Overall, 8.2% prevalence of Division II isolates (cfiA-positive) was detected in the two Slovenian tertiary care hospitals. A difference in proportion of cfiA-positive isolates between blood stream and non-blood stream specimens (14.9% vs. 7.6%; p = 0.081), was also revealed [17]. Ferløv-Schwensen and co-workers studied 444 B. fragilis group Danish clinical isolates and showed that from 1973–1980 to 2010–2015, the prevalence of antimicrobial resistance for meropenem rose from 0% to 2.5%. MALDI-TOF MS and real-time PCR identified 16 of 266 (6.0%) B. fragilis strains as Division II [44]. Overall, 7.8% (415 of 5300) B. fragilis clinical isolates studied by Cordovana et al. were found to belong to Division II, by the MALDI-TOF MS typing method, suspicious to harbor the cfiA gene in an active or inactive form. In all 70 B. fragilis strains typed by MALDI-TOF MS to belong to Division II PCR confirmed the presence of the cfiA gene. In seven B. fragilis isolates, IS elements upstream of the carbapenemase gene (IS613, IS614B, IS942, IS1169, or IS1187) were detected. All strains had a meropenem MIC ≥ 16 mg/L.
The Carba NP test detected carbapenemase activity in 6 of 29 (20.7%) Division II B. fragilis strains [45]. In a study typing 396 B. fragilis strains isolated from patients at Nagasaki University Hospital between 2006 and 2019, 8.3% harbored the cfiA gene. IS elements were found in seven cfiA-positive strains; IS612, IS1187, and IS1188 were detected in each strains, and IS612B and IS613 were detected each in two strain [46]. A review of the literature revealed that the rate of B. fragilis possessing the cfiA gene varies from 7.6% to 38.9% worldwide [14, 16, 17, 45, 46, 47]. The cfiA gene may be expressed at diverse levels, depending on the presence of IS upstream cfiA. In B. fragilis, IS942, IS1186, IS1187, IS1188, IS612, IS613, IS614, IS615, IS616, IS4351, have been related to cfiA, with varying promotion efficiency [48, 49]. In our study, IS1186 was detected in only one strain. The presence of the cfiA gene along with the IS1186 was detected in B. fragilis strain, which was susceptible to imipenem (MIC 0.125 mg/L) and resistant to meropenem (MIC 1.5 mg/L; Eucast 2022 and the latest). In the tested pool of isolates, there were cfiA-positive, but carbapenem susceptible isolates. It is known that B. fragilis with a cfiA gene can easily be converted to resistant genes by the effects of its upstream IS element, one-step mutation can allow the silent cfiA gene to be expressed [47, 48]. These results are in line with other European antimicrobial susceptibility studies [10, 17, 44, 48, 50, 51, 52, 53].
Phenotypic testing with the Carba NP test is a viable alternative for genetic technics for the presence of the cfiA gene in Bacteroides spp. The result of the Carba NP test together with the antibiogram allows to predict the effectiveness of therapy [28, 54].
In our study, the Carba NP test was positive for all cfiA-positive isolates including two strains phenotypically sensitive to meropenem with low MIC values (0.5 and 1 mg/L). In those two cases, low carbapenemase gene expression may occur. A clinical important issue is the possible conversion of meropenem-sensitive strains to resistant strains during therapy.
These results should prompt a discussion on whether carbapenem treatment is warranted when a strain of B. fragilis is cfiA-positive but phenotypically susceptible to meropenem. To date, there is little data on the clinical implications of such microbiological findings. It seems reasonable for clinicians to be cautious about treating infections with carbapenems, even if the isolate is phenotypically susceptible. Follow-up cultures monitoring the MIC of meropenem, to detect a potential increase in MIC values during treatment of the patient, are warranted [10, 17]. Javeriva and co-authors advise against amoxicillin or carbapenem therapy when Division II isolates are identified in their clinical centers [17]. This problem was also discussed by Hashimoto et al. who pointed out the importance of evaluating the use of meropenem as empirical therapy for Bacteroides sp. infections, considering the emergence of carbapenem resistance during treatment [55].
The need to monitor antibiotic susceptibility translates into biotechnological progress. Methods based on the mass spectrometry technique, not only precisely identify anaerobic bacteria, new versions of the software allow for the detection of resistance mechanisms and may be more sensitive and accurate than phenotypic methods [29, 45]. The sensitivity and specificity of the MALDI-TOF bacterial subtyping to detect cfiA in B. fragilis were 100.0 and 99.7%, respectively. Researchers find that the combination of MALDI-TOF MS and the Carba NP assay can be applied in diagnostic clinical laboratory for rapid identification of.
B. fragilis with IS element-activated cfiA gene [45, 46, 54]. It is cheaper and quicker than gene detection, which is a key factor in routine microbiological diagnostics.
There are several limitations to our study that need to be highlighted. First, it was a retrospective analysis that relied primarily on microbiological data and partial clinical data.
We lacked complete medical and treatment stories of the patients, specifically regarding their history of antibiotic therapy. This is particularly relevant concerning carbapenem therapy, which is a known selection factor for the expression of cfiA gene in Division II strains. Second, the study did not utilize more sensitive molecular methods such as Next Generation Sequencing (NGS) to explore carbapenem resistance in cfiA-negative B. fragilis isolates.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article Phenotypic and genotypic identification of carbapenem resistance in Bacteroides fragilis clinical strains and its supplementary materials.
References
Yekani M, Rezaee MA, Beheshtirouy S, Baghi HB, Bazmani A, Farzinazar A, Memar MY, Sóki J (2022) Carbapenem resistance in Bacteroides fragilis: a review of molecular mechanisms. Anaerobe 76:102606. https://doi.org/10.1016/j.anaerobe.2022.102606
Wexler NM (2007) Bacteroides: The good, the bad, and the nitty-gritty. Clin Microbiol Rev 20:593–621. https://doi.org/10.1128/cmr.00008-07
Kierzkowska M, Majewska A, Dobrzaniecka K, Sawicka-Grzelak A, Mlynarczyk A, Chmura A, Durlik M, Deborska-Materkowska D, Paczek L, Mlynarczyk G (2014) Blood infections in patients treated at transplantation wards of a clinical hospital in Warsaw. Transplant Proc 46:2589–2591. https://doi.org/10.1016/j.transproceed.2014.08.024
Elsaghir H, Reddivari AKR (2022) Bacteroides fragilis. [Updated 2022 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK553032/
Kierzkowska M, Majewska A, Młynarczyk G (2020) Anaerobic bacteria as a challenge for modern medical microbiological diagnostics. Med Og Nauk Zdr 26:360–365
Kierzkowska M, Pędzisz P, Babiak I, Janowicz J, Kulig M, Majewska A, Sawicka-Grzelak A, Młynarczyk G (2018) Difficulties in identifying the bacterial species from the genus Clostridium in a case of injury-related osteitis. Folia Microbiol (Praha) 63:533–536. https://doi.org/10.1007/s12223-018-0597-0
Nagy E, Boyanova L, Justesen US (2018) How to isolate, identify and determine antimicrobial susceptibility of anaerobic bacteria in routine laboratories. Clin Microbiol Infect 24:1139–1148. https://doi.org/10.1016/j.cmi.2018.02.008
Sood A, Ray P, Angrup A (2022) antimicrobial susceptibility testing of anaerobic bacteria: in routine and research. Anaerobe 75:102559. https://doi.org/10.1016/j.anaerobe.2022.102559
Ayesha BB, Gachinmath S, Sobia C (2022) Isolation of obligate anaerobes from clinical samples received for routine bacterial culture and sensitivity: a cross sectional study. Iran J Microbiol 14:145–155
Rong SMM, Rodloff AC, Stingu CS (2021) Diversity of antimicrobial resistance genes in Bacteroides and Parabacteroides strains isolated in Germany. J Glob Antimicrob Resist 24:328–334. https://doi.org/10.1016/j.jgar.2021.01.007
Kozhakhmetova S, Zholdybayeva E, Tarlykov P, Atavliyeva S, Syzdykov T, Daniyarov A, Mukhtarova K, Ramankulov Y (2021) Determinants of resistance in Bacteroides fragilis strain bfr_kz01 isolated from a patient with peritonitis in Kazakhstan. J Glob Antimicrob Resist 25:1–4. https://doi.org/10.1016/j.jgar.2021.02.022
Nagy E, Urbán E, Nord CE (2011) Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect 17:371–379. https://doi.org/10.1111/j.1469-0691.2010.03256.x
Snydman DR, Jacobus NV, McDermott LA, Ruthazer R, Golan Y, Goldstein EJ, Finegold SM, Harrell LJ, Hecht DW, Jenkins SG, Pierson C, Venezia R, Yu V, Rihs J, Gorbach SL (2007) National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the united states from 1997 to 2004. Antimicrob Agents Chemother 51:1649–1655. https://doi.org/10.1128/aac.01435-06
Gao Q, Wu S, Xu T, Zhao X, Huang H, Hu F (2019) Emergence of carbapenem resistance in Bacteroides fragilis in China. Int J Antimicrob Agents 53:859–863. https://doi.org/10.1016/j.ijantimicag.2019.02.017
Ulger Toprak N, Akgul O, Bilgin H, Ozaydin AN, Altinkanat Gelmez G, Sayin E, Sili U, Korten V, Soyletir G (2021) Frequency and associated factors for carbapenem-non-susceptible Bacteroides fragilis group bacteria colonization in hospitalized patients: case control study in a university hospital in turkey. Indian J Med Microbiol 39:518–522. https://doi.org/10.1016/j.ijmmb.2021.03.018
Sóki J, Eitel Z, Urbán E, Nagy E (2013) Molecular analysis of the carbapenem and metronidazole resistance mechanisms of Bacteroides strains reported in a Europe-wide antibiotic resistance survey. Int J Antimicrob Agents 41:122–125. https://doi.org/10.1016/j.ijantimicag.2012.10.001
Jeverica S, Sóki J, Premru MM, Nagy E, Papst L (2019) High prevalence of division II (CfiAPositive) isolates among blood stream Bacteroides fragilis in Slovenia as determined by MALDI-TOF MS. Anaerobe 58:30–34. https://doi.org/10.1016/j.anaerobe.2019.01.011
Hecht DW (2004) Prevalence of antibiotic resistance in anaerobic bacteria: worrisome developments. Clin Infect Dis 39:92–97. https://doi.org/10.1086/421558
Gupta AP, Shenoy PA, Kumar A, Chawla K (2021) Clinico-microbiological profile of Bacteroides fragilis with focus on molecular detection of emerging resistance. Indian J Med Res 154:750–756. https://doi.org/10.4103/ijmr.IJMR_2568_19
Sóki J, Edwards R, Hedberg M, Fang H, Nagy E, Nord CE (2006) Examination of CfiA-mediated carbapenem resistance in Bacteroides fragilis strains from a European antibiotic susceptibility survey. Int J Antimicrob Agents 28:497–502. https://doi.org/10.1016/j.ijantimicag.2006.07.021
Eberly AR, Wallace MA, Shannon S, Heitman AK, Schuetz AN, Burnham CD, Jean S (2022) Development and validation of a novel anaerobic carbapenem inactivation method (Ana-CIM) for the detection of carbapenemase production in Bacteroides fragilis. J Clin Microbiol 60:0218821. https://doi.org/10.1128/jcm.02188-21
Podglajen I, Breuil J, Bordon F, Gutmann L, Collatz E (1992) A silent carbapenemase gene in strains of Bacteroides fragilis can be expressed after a one-step mutation. FEMS Microbiol Lett 7:21–29. https://doi.org/10.1016/0378-1097(92)90557-5
Schwensen SA, Acar Z, Sydenham TV, Johansson ÅC, Justesen US (2017) Phenotypic detection of the CfiA metallo-β-lactamase in Bacteroides fragilis with the meropenem-edta double-ended Etest and the rosco KPC/MBL confirm kit. J Antimicrob Chemother 72:437–440. https://doi.org/10.1093/jac/dkw436
Bogaerts P, Engelhardt A, Berhin C, Bylund L, Ho P, Yusof A, Glupczynski Y (2008) Evaluation of a new meropenem-EDTA double-ended etest strip for the detection of the CfiA metallo-beta-lactamase gene in clinical isolates of Bacteroides fragilis. Clin Microbiol Infect 14:973–977. https://doi.org/10.1111/j.1469-0691.2008.02065.x
Dortet L, Poirel L, Errera C, Nordmann P (2014) CarbAcineto NP test for rapid detection of carbapenemase-producing Acinetobacter spp. J Clin Microbiol 52:2359–2364. https://doi.org/10.1128/jcm.00594-14
Dortet L, Bréchard L, Poirel L, Nordmann P (2014) Impact of the isolation medium for detection of carbapenemase-producing Enterobacteriaceae using an updated version of the Carba NP test. J Med Microbiol 63:772–776. https://doi.org/10.1099/jmm.0.071340-0
Poirel L, Bonnin RA, Nordmann P (2013) Rapid identification of antibiotic-resistant bacteria: how could new diagnostic tests halt potential endemics? Expert Rev Mol Diagn 13:409–411. https://doi.org/10.1586/erm.13.30
Akyar I, Ayas M, Karatuna O, Besli Y (2018) Evaluation of the Carba NP test for the detection of carbapenemase activity in Bacteroides species. Pol J Microbiol 67:97–101. https://doi.org/10.5604/01.3001.0011.6148
Wallace MJ, Jean S, Wallace MA, Burnham CD, Dantas G (2022) Comparative genomics of Bacteroides fragilis group isolates reveals species-dependent resistance mechanisms and validates clinical tools for resistance prediction. MBio 13:e0360321. https://doi.org/10.1128/mbio.03603-21
Eucast. Clinical Breakpoints and Dosing of Antibiotics (2022). https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=464&cHash=ea8540c0fbdaa71b3bbcb3bf765239de Accessed 13 December 2022
Rennie RP, Turnbull L, Brosnikoff C, Cloke J (2012) First comprehensive evaluation of the MIC. Evaluator device compared to etest and clsi reference dilution methods for antimicrobial susceptibility testing of clinical strains of anaerobes and other fastidious bacterial species. J Clin Microbiol 50:1153–1157. https://doi.org/10.1128/jcm.05397-11
Eucast. Clinical Breakpoints and Dosing of Antibiotics (2021). https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=404&cHash=74a1f440876cb3eefb60e6ad39c622e7. Accessed 13 December 2022
Tijet N, Boyd D, Patel SN, Mulvey MR, Melano RG (2013) Evaluation of the Carba NP Test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:4578–4580. https://doi.org/10.1128/aac.00878-13
Nordmann P, Poirel L, Dortet L (2012) Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 18:1503–1507. https://doi.org/10.3201/eid1809.120355
Boente RF, Ferreira LQ, Falcão LS, Miranda KR, Guimarães PL, Santos-Filho J, Vieira JM, Barroso DE, Emond JP, Ferreira EO, Paula GR, Domingues RM (2010) Detection of resistance genes and susceptibility patterns in Bacteroides and Parabacteroides strains. Anaerobe 16:190–194. https://doi.org/10.1016/j.anaerobe.2010.02.003
Podglajen I, Breuil J, Rohaut A, Monsempes C, Collatz E (2001) Multiple mobile promoter regions for the rare carbapenem resistance gene of Bacteroides fragilis. J Bacteriol 183:3531–3535. https://doi.org/10.1128/JB.183.11.3531-3535.2001
Podglajen I, Breuil J, Collatz E (1994) Insertion of a novel DNA sequence, 1S1186, upstream of the silent carbapenemase gene cfiA, promotes expression of carbapenem resistance in clinical isolates of Bacteroides fragilis. Mol Microbiol 12:105–114. https://doi.org/10.1111/j.1365-2958.1994.tb00999.x
Yekani M, Baghi HB, Naghili B, Vahed SZ, Sóki J, Memar MY (2020) To resist and persist: important factors in the pathogenesis of Bacteroides fragilis. Microb Pathog 149:104506. https://doi.org/10.1016/j.micpath.2020.104506
Valdezate S, Cobo F, Monzón S, Medina-Pascual MJ, Zaballos Á, Cuesta I, Pino-Rosa S, Villalón P (2021) Genomic background and phylogeny of CfiA-positive Bacteroides fragilis strains resistant to meropenem-EDTA. Antibiotics (Basel) 10:304. https://doi.org/10.3390/antibiotics10030304
Cao Y, Wang Z, Yan Y, Ji L, He J, Xuan B, Shen C, Ma Y, Jiang S, Ma D, Tong T, Zhang X, Gao Z, Zhu X, Fang H, JY C, Hong J (2021) Enterotoxigenic Bacteroides fragilis promotes intestinal inflammation and malignancy by inhibiting exosome-packaged Mir-149–3p. Gastroenterology 161:1552–1566. https://doi.org/10.1053/j.gastro.2021.08.003
Liu QQ, Li CM, Fu LN, Wang HL, Tan J, Wang YQ, Sun DF, Gao QY, Chen YX, Fang JY (2020) Enterotoxigenic Bacteroides fragilis induces the stemness in colorectal cancer via upregulating histone demethylase JMJD2B. Gut Microbes 12:1788900. https://doi.org/10.1080/19490976.2020.1788900
Scott N, Whittle E, Jeraldo P, Chia N (2022) A systemic review of the role of enterotoxic Bacteroides fragilis in colorectal cancer. Neoplasia 29:100797. https://doi.org/10.1016/j.neo.2022.100797
Snydman DR, Jacobus NV, McDermott LA, Golan Y, Goldstein EJ, Harrell J, Jenkins S, Newton D, Pierson C, Rosenblatt J, Venezia R, Gorbach SL, Queenan AM, Hecht DW (2011) Update on resistance of Bacteroides fragilis group and related species with special attention to carbapenems 2006–2009. Anaerobe 17:147–151. https://doi.org/10.1016/j.anaerobe.2011.05.014
Ferløv-Schwensen SA, Sydenham TV, Hansen KCM, Hoegh SV, Justesen US (2017) Prevalence of antimicrobial resistance and the cfia resistance gene in Danish Bacteroides fragilis group isolates since 1973. Int J Antimicrob Agents 50:552–556. https://doi.org/10.1016/j.ijantimicag.2017.05.007
Cordovana M, Kostrzewa M, Sóki J, Witt E, Ambretti S, Pranada AB (2018) Bacteroides fragilis: a whole Maldi-based workflow from identification to confirmation of carbapenemase production for routine laboratories. Anaerobe 54:246–253. https://doi.org/10.1016/j.anaerobe.2018.04.004
Kawamoto Y, Kosai K, Ota K, Uno N, Sakamoto K, Hasegawa H, Izumikawa K, Mukae H, Yanagihara K (2021) Rapid detection and surveillance of CfiA-positive Bacteroides fragilis using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Anaerobe 72:102448. https://doi.org/10.1016/j.anaerobe.2021.102448
Wang Y, Han Y, Shen H, Lv Y, Zheng W, Wang J (2020) Higher prevalence of multi-antimicrobial resistant Bacteroides spp. strains isolated at a tertiary teaching hospital in China. Infect Drug Resist 13:1537–1546. https://doi.org/10.2147/idr.S246318
Sóki J (2013) Extended role for insertion sequence elements in the antibiotic resistance of Bacteroides. World J Clin Infect Dis 3:1–12. https://doi.org/10.5495/wjcid.v3.i1.1
Litterio MR, Cejas D, Gutkind G, Radice M (2017) Identification of CfiA coding genes in Bacteroides fragilis isolates recovered in Argentina. Inconsistencies in CfiA organization and nomenclature. Anaerobe 48:257–261. https://doi.org/10.1016/j.anaerobe.2017.10.003
Székely E, Eitel Z, Molnár S, Szász IÉ, Bilca D, Sóki J (2015) Analysis of Romanian Bacteroides isolates for antibiotic resistance levels and the corresponding antibiotic resistance genes. Anaerobe 31:11–14. https://doi.org/10.1016/j.anaerobe.2014.09.001
Sóki J, Wybo I, Hajdú E, Toprak NU, Jeverica S, Stingu CS, Tierney D, Perry JD, Matuz M, Urbán E, Nagy E (2020) A Europe-wide assessment of antibiotic resistance rates in Bacteroides and Parabacteroides isolates from intestinal microbiota of healthy subjects. Anaerobe 62:102182. https://doi.org/10.1016/j.anaerobe.2020.102182
Kierzkowska M, Majewska A, Mlynarczyk G (2020) Trends and impact in antimicrobial resistance among Bacteroides and Parabacteroides species in 2007–2012 compared to 2013–2017. Microb Drug Resist 26:1452–1457. https://doi.org/10.1089/mdr.2019.0462
Hansen KCM, Schwensen SAF, Henriksen DP, Justesen US, Sydenham TV (2017) Antimicrobial resistance in the Bacteroides fragilis group in faecal samples from patients receiving broad-spectrum Antibiotics. Anaerobe 47:79–85. https://doi.org/10.1016/j.anaerobe.2017.04.013
Ho PL, Yau CY, Ho LY, Chen JHK, Lai ELY, Lo SWU, Tse CWS, Chow KH (2017) Rapid detection of cfia metallo-β-lactamase-producing Bacteroides fragilis by the combination of MALDI-TOF MS and Carba NP. J Clin Pathol 70:868–873. https://doi.org/10.1136/jclinpath-2017-204335
Hashimoto T, Hashinaga K, Komiya K, Hiramatsu K (2023) Prevalence of antimicrobial resistant genes in Bacteroides spp. isolated in Oita Prefecture. Japan J Infect Chemother 29:284–288. https://doi.org/10.1016/j.jiac.2022.11.011
Funding
This study was financially supported by Medical University of Warsaw with Student Mini-Grant (1M20/1/M/MG/N/20/20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Edited by Volkhard A.J. Kempf.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kierzkowska, M., Majewska, A., Karłowicz, K. et al. Phenotypic and genotypic identification of carbapenem resistance in Bacteroides fragilis clinical strains. Med Microbiol Immunol 212, 231–240 (2023). https://doi.org/10.1007/s00430-023-00765-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-023-00765-w