Abstract
The causes of cardiac inflammation during the COVID-19 pandemic are manifold and complex, and may have changed with different virus variants and vaccinations. The underlying viral etiology is self-evident, but its role in the pathogenic process is diverse. The view of many pathologists that myocyte necrosis and cellular infiltrates are indispensable for myocarditis does not suffice and contradicts the clinical criteria of myocarditis, i.e., a combination of serological evidence of necrosis based on troponins or MRI features of necrosis, edema, and inflammation based on prolonged T1 and T2 times and late gadolinium enhancement. The definition of myocarditis is still debated by pathologists and clinicians. We have learned that myocarditis and pericarditis can be induced by the virus via different pathways of action such as direct viral damage to the myocardium through the ACE2 receptor. Indirect damage occurs via immunological effector organs such as the innate immune system by macrophages and cytokines, and then later the acquired immune system via T cells, overactive proinflammatory cytokines, and cardiac autoantibodies. Cardiovascular diseases lead to more severe courses of SARS-CoV‑2 disease. Thus, heart failure patients have a double risk for complicated courses and lethal outcome. So do patients with diabetes, hypertension, and renal insufficiency. Independent of the definition, myocarditis patients benefitted from intensive hospital care, ventilation, if needed, and cortisone treatment. Postvaccination myocarditis and pericarditis affect primarily young male patients after the second RNA vaccine. Both are rare events but severe enough to deserve our full attention, because treatment according to current guidelines is available and necessary.
Zusammenfassung
Die Ursachen für eine kardiale Entzündung während der COVID-19-Pandemie sind komplex und vielfältig. Sie waren durch verschiedene Virusvarianten und Impfungen Änderungen unterworfen. Die Definition einer akuten Myokarditis ist weiterhin kontrovers zwischen Pathologen und Ärzten, die sich auf unterschiedliche diagnostische Kriterien beziehen. Für viele Pathologen gehören die Myozytolyse und Zellinfiltrate zu jeder Form von Myokarditis, Kliniker stützen sich hingegen auf Serummarker wie das hochsensitive (hs‑)Troponin für Nekrose und das NT-proBNP für die Herzinsuffizienz sowie die MRT-Kriterien einer verlängerten T1- und T2-Zeit für Ödem, Nekrose und Entzündung sowie einer späten Gadoliniumanreicherung. Wir haben gelernt, dass Myokarditis und Perikarditis vom Virus über direkte Viruseinwirkung und Zugang über den ACE2-Rezeptor Myokard zerstören kann. Indirekter Schaden entsteht über die immunologischen Effektororgane wie das angeborene Immunsystem durch Makrophagen und Zytokine und dann später über das erworbene Immunsystem durch die T‑Lymphozyten, durch kardiale Autoantikörper oder ungebremste proinflammatorische Zytokine. – Herz-Kreislauf-Erkrankungen tragen zu schwereren Verläufen, auch mit tödlichem Ausgang, bei einer Coronaviruserkrankung bei. Das Risiko bei Herzinsuffizienz und Diabetes ist doppelt so hoch, auch bei Hypertonie und Niereninsuffizienz. Unabhängig von der jeweiligen Definition einer kardialen Entzündung oder Myokarditis profitieren die Patienten von einer intensivmedizinischen Behandlung, von Beatmung bei Virusbefall der Lunge und einer Kortisontherapie. Myokarditis und Perikarditis infolge einer Impfung betreffen bevorzugt junge Männer nach der 2. Impfung. Diese Nebenwirkungen sind selten, aber dennoch schwerwiegend genug und verdienen unsere ungeteilte Aufmerksamkeit. Denn hierfür gibt es leitlinienkonforme therapeutische Empfehlungen, die umgesetzt werden sollten.
Similar content being viewed by others
In October 2019, a group of international experts were invited to Wuhan, China, to lecture at an international conference on fulminant myocarditis. This particular form of cardiac inflammation describes a special clinical phenotype of myocarditis, the underlying cause of which has remained a matter of uncertainty and of discussion [1]. It is part of the clinical spectrum of acute myocarditis, but was neither specifically mentioned in the histologically based Dallas criteria [2], nor the report of the World Heart Federation (WHF)/ISFC (International Society and Federation of Cardiology) classification of cardiomyopathies [3]. According to the WHF Consensus Classification on myocarditis [4], it falls within the histopathological entity of acute myocarditis. However, its etiology is multifold and its pathogenesis remains unclear in all cases that do not undergo myocardial tissue analysis. It is in fact a clinical syndrome in “search of its etiological diagnosis but with therapeutical options” [5].
After the world became aware of the outbreak of the COVID-19 pandemic 2 months later, which probably originated from a food market in Wuhan, we invited the organizers of this symposium, professors Dao Wang and Chen Chen, to report their first observations [6]. Consequently, we also asked the coeditors of HERZ/Cardiovascular Diseases and expert scientists in Germany to summarize in viewpoint contributions their initial experience and their assessment of the evolving pandemic [7]. Of particular interest were at that time public health issues [8,9,10], early comments on the pathogenetic aspects of pulmonary and cardiac involvement [11,12,13], and the management of patients during emergencies and in intensive care units [14,15,16].
Meanwhile, national and international medical societies have developed guidelines on COVID-19, which also include cardiological aspects. The European Society of Cardiology (ESC) has developed what was named “not a guideline but a guidance” [17, 18], which was designed as an orientation aid for physicians in the coronavirus disease 2019 (COVID-19) pandemic. A total of 62 European cardiologists as authors and 29 further experts as reviewers have contributed to this document. The German College of General Practitioners and Family Physicians (DEGAM) issued a guideline directed to the general practitioner [19].
Similar guidelines or guidance documents have been commented on both in the United States [20] and Europe [21].
Three years later, it is appropriate to take stock of our experience. We realize that some questions posed 3 years ago have been cleared, but others remain unresolved.
This review focusses on different forms of myocarditis and pericarditis associated with SARS-CoV‑2 infection and vaccination as well as on the impact on management and outcome.
The virus, its mutants and variants
The SARS-CoV‑2 pandemic started with the original Wuhan virus. The original virus was used for the first mRNA vaccines from BioNTech and Moderna. Numerous mutants and variants have been discovered since then. Several of them were named according to the countries in which they were first found. Figure 1 sums up the variant that were of epidemic relevance. Today, the most frequent variant is Omicron, which appears to be more infective but less severe than the earlier variants.
From the pandemic to the endemic: declining incidence
By 15 March 2023 the Robert Koch Institute reported for Germany that 38,276,190 individuals have been infected over the 3‑year period by SARS-CoV‑2. Only 69,345 patients tested positive on that day. The 7‑day incidence of COVID-19 was 47.4 per 100,000 inhabitants (Fig. 2a); the reported fatal cases were 169,345 [22]. This trend was interpreted as the transition from the global pandemic to an endemic situation.
a Declining number of reported infections. The 7‑day incidence: number of infected persons with SARS-CoV‑2 per 100,000 inhabitants from 2020 to 2023 (© Robert Koch Institute, https://experience.arcgis.com/experience/478220a4c454480e823b17327b2bf1d4). b Occupation of intensive care beds in Germany by number from March 2020 to 2023 (light blue). On top is the occupancy with non-COVID patients with critical illness or postoperative intensive care (© Statista 2023 [23])
The declining severity of the Omicron variant is reflected by the decrease in the number of occupied beds in intensive care units in Germany despite the obvious increase in infectivity of the Omicron variant, when compared to previous variants (Fig. 2b).
The coronavirus pandemic struck all countries in the world, even those that followed a strict non-COVID policy like China. Worldwide 759,407,939 individuals were reported to have suffered from SARS-CoV‑2, 6,866,421 patients died. Many countries are now on the way back to normality. Consequently, the rule to wear face masks in public was abandoned after 3 years of the pandemic, even in China, except for hospitals and medical practices.
Myocarditis vs. cardiac inflammation or myocardial injury
In the first 2 years of the pandemic, information on cardiac involvement was primarily based on case reports.
Kawakami et al. [24] reviewed 201 cases from different pathological institutions and summarized: From 192 autopsy and nine biopsy cases, only nine (4.5%) had active myocarditis (infiltrate plus myocyte necrosis according to the Dallas criteria; [3, 4, 25]). Their report deliberately excluded necropsy cases with borderline myocarditis, which was defined as sparse infiltrate, no necrosis. They counted these cases as non-myocarditis. In his own series with 16 patients from Bergamo (Italy), which was the Italian region hit hardest during the early phase of the pandemic, they could not identify any patient with active myocarditis. His final assessment attributed death in 13 patients only to diffuse alveolar damage (pulmonary failure), and in three cases to acute myocardial infarction. The association with a pulmonary thrombus was frequent (50%). When looking closer into their data, however, the inclusion of borderline myocarditis (infiltrate without overt necrosis) would have changed their insight into cardiac inflammation dramatically. Epicardial infiltration was present in 10 patients, lymphocytes and monocytes in the myocardial tissue samples were seen in five of the 16 patients, and, most remarkably, pericardial effusions (> 50 ml) were noted in 15 patients (94%). Thus, myocarditis (= lymphocyte and/or macrophage infiltrations with necrosis) and cardiac inflammation without necrosis (cellular infiltrate without myocyte necrosis, but with epicarditis and pericardial effusion) classified as no myocarditis remain a hardly understandable definition and, as such, a matter of debate. By applying the WHF criteria of myocarditis, which emphasize the quantitative aspect of the cellular infiltrate as more than 14 lymphocytes + macrophages/mm2 [4, 5], their review should have included the borderline type within the spectrum of myocarditis (Table 1; [26]). These quantitative WHF criteria have been part of the ESC position statement of myocarditis [27] and a recent review on inflammatory cardiomyopathy (Fig. 3; [28]).
To examine some of Kawakami and colleagues’ 201 cases in more detail is informative (Table 2).
Sala et al. [29] identified active myocarditis in a patient with the phenotype of a reverse takotsubo cardiomyopathy during the pandemic with heart failure, but could not detect viral DNA or RNA. This depicts a diagnostic dilemma even in a pandemic, in which the assumption of coronaviral etiology determined the line of thinking. Tavazzi et al. [30] reported on a patient with only a sparse infiltrate and demonstrated by electron microscopy that the virus was primarily in interstitial and endothelial cells as well as in macrophages (Fig. 4). The budding of the virus from an interstitial cell is impressive.
Borderline myocarditis in cardiogenic shock in a 69-year-old male patient. a Sparse infiltrate by CD45R0-positive cells (brown spots), no myocytolysis, × 200. b Budding virus from an interstitial cell (red arrow) at electron microscopy. Scale bar: 50 nm (with permission from [30])
Escher et al. [30] nicely showed the action of natural killer (NK) cells by positive perforin staining in the vicinity of a small vessel. Basso et al. [32] impressed us with numerous tissue sections showing dense infiltrates and necrosis characteristic of active myocarditis in three patients and samples with scars after infection (Fig. 5). But the other 18 samples had only infiltrates and no obvious necrosis, and thus did not qualify for myocarditis. Instead, they would have qualified for chronic or borderline myocarditis according to the Dallas criteria or the WHF definition. Remarkably, infiltrates were also identified in the epicardial layer (Fig. 6) and the pericardium. When analyzing 100 German patients immediately after recovery from coronavirus infection, sparse infiltrates, some with little necrosis, were reported. Their cardiac magnetic resonance imaging (MRI) had demonstrated myocarditis and pericarditis in most cases [35].
Lymphocytic myocarditis in SARS-CoV-2-positive necropsy cases. a Multifocal lymphocytic myocarditis in a 64-year-old male patient; arrow shows infiltrate + myocytolysis. H&E, × 200. b Focal myocardial lymphocytic infiltration with myocyte injury (arrow) in a 70-year-old man. H&E, × 400. c Biventricular multifocal lymphocytic myocarditis (arrows) with myocyte injury in a 64-year-old man, who developed atrial fibrillation 2 days before death. Double immunostaining CD4 brown/CD8 red, × 400 (with permission from [33])
Infiltrating cells at the epicardium and the adjacent myocardial tissue. Focal lymphocytic epi- and pericarditis (arrows) in a 66-year-old man with focal myocardial inflammation, but without myocyte injury (arrowheads). a H&E, × 400; b CD8 immunostaining, × 400 (with permission from [33])
Necropsy data from a large study of 277 COVID-19 patients by Halushka et al. gave similar results: Myocarditis and pericarditis were ascertained in 7.2% and in 6.9%, respectively, of their pathoanatomical data set [36].
The data above together show the divergence in the pathologists’ interpretations of active myocarditis, on the one hand, and the use of wording such as “myocardial injury” or “inflammatory changes” to avoid the term “myocarditis,” on the other hand, by clinicians. This divergence becomes even more obvious when clinical definitions of myocarditis are used with positive biomarker for necrosis, e.g., hs-troponins, and heart failure, such as N‑terminal pro-B-type natriuretic peptide (NT-proBNP) and cardiac MRI. Ammirati et al. [37] calculated the prevalence of 112 patients with suspected acute myocarditis (AM) from 56,963 patients hospitalized for COVID-19 to be 2.4 per 1000 hospitalizations when considering definite/probable myocarditis cases and 4.1 per 1000 when considering also possible acute myocarditis patients. Their diagnosis of myocarditis was based on endomyocardial biopsies or increased troponin levels plus typical signs of acute myocarditis (AM) on cardiac MRI. With this composite view they identified 97 patients with possible AM, and among them, 54 patients with definite/probable AM supported by endomyocardial biopsy in 17 (31.5%) or magnetic resonance imaging in 50 patients (92.6%).
When summarizing 48 case reports of 56 patients with pericarditis, the diagnosis was made mostly on the basis of clinical criteria: It showed a preponderance of men in 59% of cases with a mean age of 53 ± 18 years. Pneumonia was diagnosed in 41%, pleuritis in 21% of cases. Symptoms compatible with pericarditis such as precordial chest pain or typical EKG changes were detected in 66% of cases. Echocardiography demonstrated pericardial effusion in 86%, whereby tamponade was seen in 28 of the 56 patients. When the pericardial fluid was tested for SARS-CoV‑2 by polymerase chain reaction (PCR), nine patients were positive.
In Germany, 176,137 hospitalizations (52.3% males, 53.6% aged ≥ 70 years) with confirmed COVID-19 infection were coded in 2020. Only 226 of them (0.01%) were coded to have myocarditis, which corresponds to an incidence of 1.28 per 1000 hospitalization cases [24]. Remarkably, the absolute numbers of myocarditis patients increased for those with SARS-CoV‑2, whereas the relative number decreased with the age of the patients. This converse relationship points to the fact that COVID-19 patients with myocarditis were younger. Case fatality from myocarditis in hospital was 1.3-fold higher in COVID-19 patients with or without myocarditis (24.3% vs. 18.9%, p = 0.012). Risk factors for myocarditis in COVID-19 were age < 70 years, male sex, pneumonia, and multisystemic inflammatory COVID-19 infection. Myocarditis was independently associated with increased case fatality.
Pathogenesis of SARS-CoV2 infection
In vitro studies indicate that SARS-CoV‑2 can infect isolated human iPS-cardiomyocytes, cardiospheres, human heart slices, and a human heart biopsy directly in an angiotensin-converting enzyme 2 (ACE2)- and cathepsin-dependent manner [38]. SARS-CoV‑2 infection of cardiomyocytes was inhibited in this in vitro setting by the antiviral drug remdesivir.
In patients the pathogenetic process starts with surface adherence of the single-stranded RNA virus SARS-CoV‑2 in the respiratory tract. During viremia, the virus can also enter other organs and cells expressing an ACE2 receptor [39], whereby coactivation by transmembrane protease serine subtype 2 (TMPRSS) enables the uncoating of the virus and its incorporation into the target cell [40]. The following steps of viral replication can be appreciated best in Fig. 7, which also depicts the junctions to interventions by medical therapy.
SARS-CoV‑2 cell entry and replication. Medical intervention by drugs. ACE2 angiotensin-converting enzyme 2, TMPRSS2 transmembrane protease serine subtype 2 (with permission from [21])
The pivotal role of ACE2 in the pathogenesis of COVID-19-induced myocardial inflammation has been underlined recently [41, 42]. ACE2 plays a well-known role as an antifibrotic, antihypertrophic, and vasodilatory mediator of the cardiovascular hormone system. It also acts as an entry receptor for SAR-CoV‑2 and serves as a link between the virus and the immune response with inflammation attributed to proinflammatory cytokines. SARS-CoV‑2 can upregulate a disintegrin and metalloprotease 17, which in turn is involved in myocardial injury.
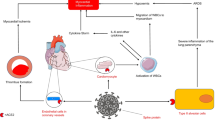
In addition to direct damage to the myocardium, SARS-CoV‑2 exerts indirect effects on the immune system. Antigen-presenting cells (APCs) process and deliver viral antigens to naive T cells, which then become activated, cytotoxic CD8+ T cells. They amplify the inflammatory response, known as a cytokine storm, which is the result of the overproduction of proinflammatory mediators, such as interleukin (IL)-6, interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. Within the innate immune system, toll-like receptors (TLRs) may be activated and permit further entry of the virus into ACE-receptor positive cells (Fig. 8; [11]).
PHASES in immunopathology and FACES in clinical presentation. GM-CSF granulocyte–macrophage colony-stimulating factor, IL interleukin, IFN interferon, M‑CSF macrophage colony-stimulating factor, MODS multiple organ dysfunction syndrome, Th T helper, TNF tumor necrosis factor (with permission from [11])
Clinical phenotype and outcome
The patient with inflammatory cardiomyopathy or perimyocarditis can present with various symptoms. To those listed in Table 3, patients with COVID-19 can be added in all five categories.
Ammirati et al. [37] found in 112 patients with all forms of acute myocarditis, from possible to definite cases, a mean age of 38 years and a male preponderance of 61.2%. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively). Overall, 31 cases (57.4%) occurred in the absence of COVID-19-associated pneumonia. A total of 21 (38.9%) had a fulminant presentation requiring inotropic support or temporary mechanical circulatory support. The composite of in-hospital mortality or temporary mechanical circulatory support occurred in 20.4%. At 120 days, the estimated mortality was 6.6%, 15.1% in patients with associated pneumonia versus 0% in patients without pneumonia (p = 0.044). During hospitalization, left ventricular ejection fraction, assessed by echocardiography, improved from a median of 40% on admission to 55% at discharge (n = 47; p < 0.0001). Corticosteroids were frequently administered (55.5%) and contributed to improvement.
Imaging for COVID-19 cardiac inflammation
Echocardiography
It is possible to show sequelae during and after COVID-19 infection such as cardiac enlargement, dyssynchrony in wall motion, pulmonary hypertension (TAPSE), pump failure, or pericardial effusion. This is important but only indirect evidence of cardiac involvement of SARS-CoV-2-induced cardiac inflammation.
Cardiac MRI
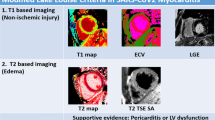
From various studies, those including a comparative analysis with biomarkers for necrosis (hs-troponin) and for heart failure (NT-proBNP) in the serum and with endomyocardial biopsies are particularly informative. In their landmark study of 100 patients after recovery from COVID-19 with a previous positive swab test, Puntmann et al. [35] found persistent elevated hs-troponin (> 3 pg/mL) in 71 patients. The following diagnostic criteria for myocarditis were used: late gadolinium enhancement (LGE) codes for scar or inflammation, prolonged native T1 time codes for edema, prolonged native T2 time codes for necrosis. If both native T1 and native T2 times were prolonged, inflammation was assumed. Figure 9 thus demonstrates their results:
-
1.
Elevated hs-troponin (> 3 pg/mL), a serum marker of necrosis, was found in 71 patients.
-
2.
In cardiac MRI, prolonged T1 time coded for edema in 71 patients, and
-
3.
Prolonged T2 time coded for necrosis in 60 patients.
-
4.
Both, T1 and T2 were prolonged together in 60 patients, which coded for inflammation.
-
5.
Late gadolinium enhancement (LGE) coding for scar or inflammation was found in 32 patients.
-
6.
In the endomyocardial biopsy (EMB) a sparse infiltrate by CD45R0-positive cells was described, which coded for borderline or chronic myocarditis in COVID-19 patients. Of note, no virus was found in myocytes.
COVID-19 and perimyocardial inflammation in COVID-19 patients: MRI results. Design: 100 patients after recovery from COVID-19 with previous positive swab test. Inclusion criteria: (1) virus positivity in original swab, (2) clinical myocarditis with elevated troponin. Results: (1) Elevated hs-troponin (> 3 pg/mL in 71 patients). (2) MRI: late gadolinium enhancement (LGE) at epi- (yellow arrowheads) and pericardium (white arrowheads) in 32 patients (a). (3) MRI with prolonged native T1 (edema) in 71 patients (b) and prolonged native T2 (necrosis) in 60 patients (c). (4) Biopsy: sparse infiltrate (CD45R0-positive cells, brown spots, d). No virus found in myocytes (from [35] published under the terms of the CC-BY License. © 2020 Puntmann VO et al. JAMA Cardiology)
Similar observations were made by Tanacli R et al. in a smaller series [43].
Long COVID and post-COVID
After SARS-CoV‑2 infection the acute disease may last up to 4 weeks with general symptoms such as fatigue, dyspnea, dysosmia (olfactory disturbance), dysgeusia (taste disorder), headache, and performance limitations. If symptoms continue for the following weeks, the appropriate term is “long COVID,” if they still persist beyond the 12th week, it is “post-COVID syndrome.” About 15% of individuals after a COVID-19 infection suffer from post-COVID symptoms. To which degree, beyond these neurological symptoms, cardiac manifestations also persist and show structural cardiac defects is not yet clear. But an autoimmune process also against the heart with demonstrable antiheart antibodies could be a likely explanation [44]. Moreover, acute or chronic pericarditis has been described in 22% of 180 patients more than 12 weeks after previous infection [45]. Treatment with colchicine or prednisolone would be appropriate [46] and conform with current guidelines.
Treatment and treatment studies
Possible treatment options can be derived from Fig. 8. In addition, treatment of cardiac problems should be performed according to current guidelines on heart failure and pericardial diseases. In post-COVID myocarditis and pericarditis, recommended options are prednisolone treatment or colchicine. Immunoglobulin treatment and immunoabsorption have been undertaken, but reports on randomized studies are missing.
In Germany, a fairly large number of treatment studies have been initiated [47]. Ongoing studies are listed in Table 4.
Postvaccination myocarditis and pericarditis
Myocarditis and pericarditis after mRNA vaccination are rare events, but have recently come to the public’s attention. In the Nordic Outcome Study myocarditis of different origins was compared from 2019 to 2022: 7292 myocarditis patients older than 12 years were registered in a population of 23 million inhabitants. The incidence of myocarditis for all etiologies was only 0.0003%. Thereby, 6653 patients suffered from conventional myocarditis, which is 91.2%. Overall, 530 patients had myocarditis after COVID vaccination, which is 7.3%, and 109 patients suffered from myocarditis due to COVID infection, which is 1.5% [48].
A study from Israel sees vaccination-induced myocarditis in young men after the second vaccination as the major target [49]. Similar observations were reported for BNT162b2 mRNA (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines in the United States. These side effects have not been reported during previous pivotal studies of the vaccines [50].
It is no wonder that pericarditis as an unwanted side effect occurred at the same time and often in the same patient [51].
Conclusion
-
Myocarditis and pericarditis can be induced by the virus via different mechanisms of action: direct viral damage to the myocardium by using the ACE2 receptor, indirect damage by means of pathways of inflammation by T cells, macrophages, cytokines, and autoantibodies.
-
The definition of myocarditis is still a matter of debate between pathologists and clinicians. Each specialty favors its own criteria. There is a gap in incidence between the different diagnostic approaches.
-
Cardiovascular diseases lead to more severe courses of SARS-CoV‑2 disease. Thus, heart failure patients have a double risk for complicated courses and lethal outcome. So do patients with diabetes, hypertension, and renal insufficiency.
-
We have learned a lot, but there is still much to do in the future.
References
Cooper LT (2009) Myocarditis. N Engl J Med 360(15):1526–1538. https://doi.org/10.1056/NEJMra0800028
Aretz HT, Billingham ME, Edwards WD et al (1987) Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1:3–14
Richardson P, McKenna W, Bristow M et al (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation 93:841–842
Maisch B, Bültmann B, Factor S et al (1999) World Heart Federation consensus conferences’s definition of inflammatory cardiomyopathy (myocarditis): report from two expert committees on histology and viral cardiomyopathy. Heartbeat 4:3–4
Maisch B, Ruppert V, Pankuweit S (2014) Management of fulminant myocarditis: a diagnosis in search of its etiology but with therapeutic options. Curr Heart Fail Rep 11:166–177. https://doi.org/10.1007/s11897-014-0196-6
Chen C, Zhou Y, Wang DW (2020) SARS-CoV-2: a potential novel etiology of fulinant myocarditis. Herz 45(3):230–232. https://doi.org/10.1007/s00059-020-04909-z
Maisch B, Dörr R (2020) COVID-19—What we know and what we need to know: There are more questions than answers. Herz 45:311–312
Stang A, Standl F, Jöckel K‑H (2020) Characteristics of COVID-19 pandemic and public health health consequences. Herz 45:313–315. https://doi.org/10.1007/s00059-020-04932-0
Gitt AK, Bernhardt A, Zahn R et al (2020) The COVID-19 Registry in Rhineland-Palatinate in the context of international registry activities documenting COVID-19 outcomes. Herz 45:316–318. https://doi.org/10.1007/s00059-020-04928-2
Dörr R (2020) Protecting patients and healthcare personnel from COVID-19: considerations for practice and outpatient care in cardiology. Herz 45:319–320. https://doi.org/10.10007/s00059-020-04922-2
Maisch B (2020) SARS-CoV‑2 as potential cause of cardiac inflammation and heart failure. Is it the virus, hyperinflammation, or MODS? Herz 45:321–322. https://doi.org/10.1007/s00059-020-04925-z
Kessler T, Schunkert H (2020) Inhibitors of the renin-angiotensin system and SARS-CoV‑2 infection. Herz 45:323–324. https://doi.org/10.1007/s00059-020-04920-4
Kuck K‑H (2020) Arrhythmias and sudden cardiac death in the COVID-19 pandemic. Herz 45:325–326. https://doi.org/10.1007/s00059-020-04924-0
Thiele H (2020) Cardiovascular emergencies in the COVID-19 pandemic. Herz 45:327–328. https://doi.org/10.1007/s00059-020-04931-1
Möhlenkamp S, Thiele H (2020) Ventilation of COVID-19 patients in intensive care-units. Herz 45:329–331. https://doi.org/10.1007/s00059-020-04923-1
Ge J (2020) Extracorporeal membrane oxygenation in the treatment of respiratory failure during COVID-19. Herz 45:332–333. https://doi.org/10.1007/s00059-020-04927-x
The Task Force for the management of COVID-19 of the European Society of Cardiology (2022) European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic:part1—Epidemiology, pathophysiology,and diagnosis. Eur Heart J 43(11):1033–1058. https://doi.org/10.1093/eurheartj/ehab696
Task Force for the management of COVID-19 of the European Society of Cardiology (2022) ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2‑care pathways, treatment, and follow-up. Eur Heart J 43(11):1059–1103. https://doi.org/10.1093/eurheartj/ehab697
Deutsche Gesellschaft für Allgemeinmedizin (DEGAM), Blankenfeld H, Kaduszkiewicz H, Kochen MM et al (2020) Neues Coronavirus (SARSCoV-2) – Informationen für die hausärztliche Praxis. AWMF-Register-Nr. 053-054, 6. Version (www.degam-leitlinien.de)
Hendren NS, Drazner MH, Bozkurt B, Cooper LT Jr (2020) Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 141(23):1903–1914. https://doi.org/10.1161/CIRCULATIONAHA.120.047349
Maisch B (2021) The ESC recommendations for COVID-19-no guideline, but a learning guidance. Herz 46(1):46–55. https://doi.org/10.1007/s00059-020-05006-x
https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html. Accessed 15 Mar 2023
https://de.statista.com/themen/6018/corona. Accessed 16 Mar 2023
Kawakami R, Sakamoto A, Kawai K et al (2021) Pathological evidence for SARS-CoV‑2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol 77(3):314–325. https://doi.org/10.1016/j.jacc.2020.11.031
Aretz HT (1987) Myocarditis: the Dallas criteria. Hum Pathol 18:619–624
Maisch B, Portig I, Ristic A et al (2000) Definition of inflammatory cardiomyopathy(Myocarditis): On the way to consensus. A status report. Herz 25(3):200–209
Caforio AL, Pankuweit S, Arbustini E et al (2013) Current state of knowledge on aetiology, diagnosis, management an therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34:2636–2648
Maisch B, Pankuweit S (2020) Inflammatory dilated cardiomyopathy. Etiology and clinical management. Herz 45:221–229
Sala S, Peretto G, Gramegna M et al (2020) Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV2 respiratory infection. Eur Heart 31:1861–1862
Tavazzi G, Pellegrini C, Mauretti M et al (2020) Myocardial localiization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 22:911–915
Escher F, Pietsch H, Aleshcheva G et al (2020) Detection of viral SARS-CoV‑2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail 7:2440–2442
Wenzel P, Kopp S, Gobel S et al (2020) Evidence of SARS-CoV‑2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res 115:1661–1663
Basso C, Leone O, Rizo S et al (2020) Pathological features of COVID-19 associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 41:3827–3835
Fox SE, Li G, Akmatbekov A et al (2020) Unexpected features of cardiac pathology in COVID-19 infection. Circulation 142:1123–1125
Puntmann VO, Careri L, Wieters I et al (2020) Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 5(11):1265–1273. https://doi.org/10.1001/jamacardio.2020.3557
Halushka MK, Vander Heide RS (2021) Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 post-mortem examinations. Cardiovasc Pathol 50:107300
Ammirati E, Lupi L, Palazzini M et al (2022) Prevalence, characteristics, and outcomes of COVID-19–associated acute myocarditis. Circulation 145:1123–1139. https://doi.org/10.1161/CIRCULATIONAHA.121.056817
Bojkova D, Wagner JUG, Shumliakivska M et al (2020) SARS-CoV‑2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res 116(14):2207–2215. https://doi.org/10.1093/cvr/cvaa267
Wang W, Xu Y, Gao R et al (2020) Detection of SARS-CoV‑2 in different types of clinical specimens. JAMA 323:1843–1844
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV‑2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Magadum A, Kishore R (2020) Cardiovascular manifestations of COVID-19 infection. Cells 9:2508. https://doi.org/10.3390/cells9112508
Pannucci P, Jefferson SR, Hampshire J et al (2023) COVID-19-induced myocarditis: Pathophysiological roles of ACE2 and toll-like receptors. Int J Mol Sci 24:5374. https://doi.org/10.3390/ijms24065374
Tanacli R, Doeblin P, Götze C et al (2021) Covid-19 vs. classical myocarditis associated myocardial injury evaluated by cardiac magnetic resonance and endomyocardial biopsy. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2021.737257
Blagova O, Lutokhina Y, Kogan E et al (2022) Chronic biopsy proven post-COVID myoendocarditis with SARS-Cov‑2 persistence and high level of antiheart antibodies. Clin Cardiol 45(9):952–959. https://doi.org/10.1002/clc.23886
Lloyd Dini F, Baldini U, Bytyci I et al (2023) Acute pericarditis as a major clinical manifestation of Long COVID-19 syndrome. Int J Cardiol 374:129–134. https://doi.org/10.1016/j.ijcard.2022.12.019
Blagova O, Yuliya L, Savina P (2023) Corticosteroids are effective in the treatment of viruspositive post-COVID myoendocarditis with high autoimmune activity. Clin Cardiol 46(3):352–354. https://doi.org/10.1002/clc.23978
Deutsches Zentrum für Infektionsforschung (DZIF) Klinische Studien. https://clinicalsite.org/~dzif/de/cat/2084. Zugegriffen: 21.03.2023
Husby A, Gulseth HL, Hovi P et al (2023) Clinical outcomes of myocarditis after SARS-CoV‑2 mRNA vaccination in four Nordic countries: Population based cohort study. BMJMED 2:e373. https://doi.org/10.1136/bmjmed-2022-000373
Mevorach D, Anis E, Cedar N et al (2021) Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med 385:2140–2149. https://doi.org/10.1056/NEJMoa2109730
Larson KF, Ammirati E, Adler ED et al (2021) Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation 144(6):506–508
Diaz GA, Parsons GT, Gering SK et al (2021) Myocarditis and pericarditis after vaccination for COVID-19. JAMA. https://doi.org/10.1001/jama.2021.13443
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B. Maisch declares that he has no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Rights and permissions
About this article
Cite this article
Maisch, B. SARS-CoV-2, vaccination or autoimmunity as causes of cardiac inflammation. Which form prevails?. Herz 48, 195–205 (2023). https://doi.org/10.1007/s00059-023-05182-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-023-05182-6