Abstract
Microcephaly is the more severe brain malformation because of Zika virus infection. Increased vulnerability of neural stem and progenitor cells to Zika infection during prenatal neurodevelopment impairs the complete formation of cortical layers. Normal development of cerebellum is also affected. However, the follow-up of apparently healthy children born to Zika exposed mothers during pregnancy has revealed other neurological sequelae. This suggests Zika infection susceptibility remains in nervous tissue after neurogenesis end, when differentiated neuronal populations predominate. The neuronal nuclear protein (NeuN) is an exclusive marker of postmitotic neurons. Changes in NeuN expression are associated with neuronal degeneration. We have evaluated immunohistochemical expression of NeuN protein in cerebral cortex, hippocampus, and cerebellum of normal and Zika-infected neonatal Balb/c mice. The highest NeuN immunoreactivity was found mainly in neurons of all cortical layers, pyramidal layer of hippocampus, granular layer of dentate gyrus and in internal granular layer of cerebellum. Viral infection caused marked loss of NeuN immunostaining in all these brain areas. This suggests neurodegenerative effects of Zika virus infection during postmitotic neuron maturation and contribute to interpretation of neuropathogenic mechanisms of Zika.
Similar content being viewed by others
Introduction
Zika virus (ZIKV) is a flavivirus transmitted by mosquitoes and was first isolated in Africa in Macacus monkeys in 1947. With the first tests in mice, its ability to infect the nervous system through intracerebral inoculation was confirmed (Dick 1952). Then, electron microscopy allowed the observation of viral particles in neurons and glial cells in brain samples from mice inoculated with ZIKV (Bell et al. 1971). For decades, only sporadic cases of Zika infection were known in humans, with imperceptible or benign signs and symptoms. With the epidemic in French Polynesia in 2013, the first cases of Guillain Barré syndrome associated with ZIKV infection were documented. In 2015, the virus arrived in the Americas through Brazil, where it was associated with a parallel increase in cases of microcephaly in children born to pregnant mothers who acquired the infection in the first months of pregnancy. In a short time, ZIKV spread throughout America and other parts of the world and acquired importance as an emerging neuropathological agent (Carod-Artal 2016; Wen et al. 2017).
Studies with animal models and cell lines of human origin established that stem cells and neural progenitor cells (NPCs) had the greatest vulnerability to infection (Li et al. 2016a; Garcez et al. 2016; Wen et al. 2017). This was confirmed by demonstrating the interaction of the noncoding regions of ZIKV with the Musashi-1 protein present in high concentrations in NPCs (Chavali et al. 2017). Mutations in some ZIKV proteins that arrived in America were also considered determinants of increased susceptibility to infection in these cells (Wen et al. 2017; Yuan et al. 2017). The tropism of ZIKV by NPCs was confirmed when adult mice were inoculated; the virus was located mainly in those areas where neurogenesis was preserved, i.e., the subventricular zone and the subgranular zone of the dentate gyrus (Li et al. 2016b). Integrin αVβ5, a molecule present in the cell membrane of NPCs, seems to be very important for the entry of ZIKV into these cells, further evidence of their greater vulnerability to infection (Wang et al. 2020). The interference of ZIKV with the cell cycle and apoptosis in NPCs has been proposed among the mechanisms leading microcephaly (Wen et al. 2017).
Although the neurobiological investigation of ZIKV infection has focused more on the early phases of neurodevelopment, due to its association with congenital Zika syndrome (CZS), which includes microcephaly, there is evidence that infection with this flavivirus acquired during the second half of pregnancy or in the first postnatal months can lead to other neurological sequelae detected in the first years of life of children born to mothers exposed to the virus in endemic areas (Lebov et al. 2018; Nielsen-Saines et al. 2019). Animal models that simulate human neurodevelopment after gestational neurogenesis are very useful for generating knowledge about the mechanisms underlying the pathogenesis of Zika beyond CZS (Ireland et al. 2020).
In this study, we used a murine model to mimic postnatal ZIKV infection (Laiton-Donato et al. 2019) to evaluate the effect of this infection in neuronal populations identified via immunohistochemistry with a neuronal differentiation marker. Neuronal nuclear protein (NeuN), initially isolated in the nervous system of mice and other vertebrates (Mullen et al. 1992) is ideal for this purpose because within the central nervous system (CNS), it is located exclusively in postmitotic neurons (Guselnikova and Korzhevskiy 2015; Sarnat 2015). NeuN has been used in the investigation of neurodevelopment and neurogenesis in adults (Sarnat 2015; Bekiari et al. 2020) as well as in histological and histopathological studies of nervous tissue (Wolf et al. 1996; Gittins and Harrison 2004). There are few references to the effect of viral infections on NeuN expression (Ghoshal et al. 2007; Rengifo et al. 2016; Mao et al. 2022) even though this protein has been used as a marker to confirm or rule out viral infections in differentiated neurons (Li et al. 2016b; Feuer et al. 2003; An et al. 2016; Yarandi et al. 2020; Geiselhardt et al. 2022; Piccirilli et al. 2023).
Materials and methods
Inoculation of the virus and management of experimental animals
BALB/c mice of both sexes (age, 20–24 h; average weight, 1.3 g) were inoculated intraperitoneally with 60 µl of a solution containing ZIKV previously isolated from a patient infected by Zika and in whom its neurotropic character had been experimentally demonstrated (Laiton-Donato et al. 2019). The viral titer obtained via Vero cell culture was 9.4 × 106 PFU/ml. We worked with two litters of five animals each accompanied by their respective mothers. One group of mice was inoculated with the virus, and the other group was inoculated with vehicle solution devoid of the virus (control). The animals were confined to a BSL-2 biosecurity room in the vivarium of the National Institute of Health (INS) under environmental and nutritional conditions in accordance with national and international ethical standards. The animal management protocol was endorsed by the Ethics Committee of the INS (Minutes No. 13, May 19, 2016). At 10 days postinoculation, when the mice showed advanced characteristics of the disease (notable loss of size and weight) and neurological signs (hypotonia, disorientation, paralysis of the hind limbs, and loss of balance), the animals were anesthetized with 50 µl of chloral hydrate (Merck Darmstadt, Germany) by intraperitoneal injection. After verifying the loss of reflexes and sensitivity to pain, a ventral longitudinal incision was made in the thorax and abdomen to expose the still-beating heart. A short needle (No. 27) was introduced into the left ventricle, directed to the aorta; the needle was coupled to a peristaltic pump tube that allowed the perfusion of 10 ml of phosphate buffer solution (PBS), then 40 ml of 4% paraformaldehyde (PFA) and finally 5 ml of PBS. Next, the brain was extracted and placed in fresh 4% PFA solution at 4 °C for less than two weeks before conducting immunohistochemistry.
Immunohistochemistry
A series of coronal brain sections from the frontal cortex and hippocampus, and sagittal sections from the cerebellum (50-µm thick) were obtained using a vibratome. The sections were collected in small Petri dishes (2.5 cm in diameter) containing PBS (the solution used for all washes). The free-floating sections were maintained in these containers in constant agitation and room temperature (20 °C) throughout the immunohistochemical procedure. After 20–24 h of washing, the sections were incubated in 0.05 M of ammonium chloride for 30 min to remove aldehyde residues and then with 3% of hydrogen peroxide for 30 min to inhibit endogenous peroxidase. Next, the sections were treated for 30 min with a solution composed of normal horse serum, bovine serum albumin and a surfactant to block nonspecific reaction sites. For the detection of NeuN protein, the sections were incubated overnight with anti-NeuN (mouse monoclonal, clone A60; Merck-Millipore, Darmstadt, Germany; 1:2500). Then, they were incubated for two hours in a solution containing anti-mouse secondary antibody (Merck-Sigma, 1:400). Next, the sections were incubated for two hours in a solution prepared with the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA, USA). For development, a diaminobenzidine (DAB) Substrate Kit with Nickel (Vector) was used. Sections that were not incubated in primary antibody or in secondary antibody were used as negative controls. The sections were placed on glass slides pre-treated with gelatin, air-dried, and mounted with Entellan (Merck). Processing free-floating sections avoids some drawbacks reported for the immunohistochemical staining of NeuN in paraffin-embedded tissues (Korzhevskii et al. 2006) and better preserves the antigen, allowing the use of a more highly diluted antibody (Rengifo et al. 2016).
Qualitative and quantitative analyses
Immunohistochemical preparations were first analyzed qualitatively to select the areas of the tissue that were immunoreactive to NeuN (NeuN+); once selected, the areas were quantitatively studied for possible ZIKV-induced changes in the neuronal cytoarchitecture and in the tissue expression of NeuN. For the cerebral cortex, NeuN+ cells were counted in 1 mm2 fields (10× objective), which included all cortical layers, using Netzmiier mesh adapted to one of the eyepieces of a Zeiss Standard-16 microscope. In the hippocampus and cerebellum, where more densely clustered areas of NeuN-immunoreactive neurons were found, quantification was carried out by optical densitometry using a Zeiss Axiophot microscope with a digital camera equipped with Capture-Pro-6 and ImageJ.
For the statistical analysis of each of the variables (cell counts and optical densitometry), data were collected from five infected animals (experimental units) with their respective controls. From each experimental unit, five sections with similar neuroanatomical characteristics were selected to perform NeuN+ cell counts and optical densitometry in the brain areas (cerebral cortex, hippocampus, and cerebellum). The data obtained were processed in InfoStat using the nonparametric Wilcoxon-Mann-Whitney U test.
Results
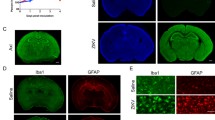
In the cerebral cortex of the controls, a uniform distribution pattern of NeuN + neurons were observed in all layers, except for layer I, which had a low number of immunopositive cells. In layer IV, there was less immunostaining of NeuN + neurons, such that a less dense strip was observed between the supragranular and infragranular cortex (Fig. 1a). In the cortex of infected animals, the loss of immunoreactivity to NeuN was notable (Fig. 1b). The images taken at higher magnification (Fig. 1c y d) revealed a decrease in immunostaining and in the amount of NeuN + cells in the mice infected with ZIKV. The counts showed a significant loss of NeuN immunoreactive neurons in ZIKV infected samples. There was an average of 2452 and 1439 NeuN+ cells per 1 mm2 of cortex in the controls and in the infected animals, respectively. The decrease in NeuN+ cells was also statistically significant for each of the six cortical layers (Table 1).
NeuN immunoreactivity in frontal cortex of 11-day-old mice. a Control mouse. NeuN+ neurons are display in all cortical layers. Layer I show few immunopositive cells (arrow) and layer IV exhibit less immunostaining (asteriks). b Zika-infected mouse. The cortex shows notable NeuN immunoreactivity loss. c, d Images at higher magnification of the supragranular cortex (layers II and III) of a control mouse c and a Zika-infected mouse d. Scale bars: 160 μm (a, b), 40 μm (c, d)
The presence of NeuN protein in the hippocampal formation was mainly concentrated in the pyramidal cell layer (CA1, CA2 and CA3) and in the granular cell layer of the dentate gyrus, where dense continuous immunoreactive bands were formed (Fig. 2a). In the samples from infected animals, banding was less intense, both in the pyramidal layer and in the granular stratum (Fig. 2b). The densitometric analysis indicated a statistically significant loss of immunoreactivity in the two areas (Table 2). In other areas of the hippocampal formation, only a few dispersed NeuN+ cells were observed. There was no immunoreactivity in the subgranular zone of the dentate gyrus of the controls or the infected mice (Fig. 2a and b).
NeuN immunoreactivity in coronal sections of hippocampal formation. a Control mouse. Hippocampus pyramidal layer (long arrows) and granular stratum of dentate gyrus (short arrows) show dense immunostaining. b Zika-infected mouse. Lower immunoreactivity in the pyramidal and granular layers is evident. The needle point indicates non-immunoreactive subgranular zone. Scale bars: 300 µm (a, b)
In the sagittal sections of the cerebellum, immunoreactivity to NeuN in the control mice was observed exclusively in the inner granular layer, with a uniform distribution pattern in the ten folia. No Purkinje cells or NeuN+ molecular layer cells were observed. There was also no immunoreactivity in the deep cerebellar nuclei or in the outer granular layer (Fig. 3a and c). In the sections of the cerebellum from infected animals, the decrease in NeuN immunostaining was marked (Fig. 3b and d). The presence of calcifications in folia VI and VII of the infected mice made reading the results difficult in some of the samples due to tissue damage and the formation of a background. The loss of immunoreactivity to NeuN in the granular layer of the cerebellum of ZIKV-infected animals was very evident and statistically significant in all the samples evaluated (Table 3).
NeuN immunoreactivity in sagittal sections of cerebellum. a Control mouse. Granular layer (short arrows) shows dense immunostaining in all folia. Note absence of immunoreactivity in the deep nuclei area (star) as well as in molecular layer and the strip corresponding to outer granular layer (long arrows). b Zika-infected mouse. Granular layer shows evident decrease in immunostaining. In folia VI and VII (arrowheads), background and tissue vacuolization are observed because it is a calcification formation zone. c, d Images at higher magnification of the molecular and granular layers in control mouse c and Zika-infected mouse d. No Purkinje nor outer granular layer immunoreactive cells are revealed. Scale bars: 600 μm (a, b), 65 μm (c, d)
Discussion
ZIKV infection in neonatal BALB/c mice
BALB/c mice showed accentuated neurological signs from the sixth day after intraperitoneal inoculation with ZIKV. We previously demonstrated the neurotropic character of the ZIKV strain used herein and the presence of viral infection by means of RT–PCR, immunohistochemistry and in situ hibridization in the nervous tissue of BALB/c mice inoculated intraperitoneally (Laiton-Donato et al. 2019; Corchuelo et al. 2021). Importantly, this work was performed using an immunocompetent mouse model because much of the research on the pathogenesis of Zika has been carried out with transgenic mice due to the lack of susceptibility of mice to ZIKV strains of Asian or African origin (Narasimhan et al. 2020). Nervous system development in neonatal mice (P0–P3) is approximately equivalent to the second and third trimesters of human gestation, when neurogenesis has already ended and the neurons are in a period of postmitotic maturation (Semple et al. 2013; Van den Pol et al. 2017). Therefore, mouse models inoculated in the postnatal phase can provide valuable information to elucidate the pathogenesis of ZIKV when the infection has been acquired in the second half of pregnancy or during the first months as neonates.
Distribution of NeuN in the brain of mice
The distribution of NeuN + cells in the cerebral cortex of 11-day-old suckling mice was like that known for adult mice and other mammals, including humans (Mullen et al. 1992; Gittins and Harrison 2004; Rengifo et al. 2016; Hight et al. 2010; Atapour et al. 2019). NeuN is present in all cortical layers. Layer I appear depopulated of cells because the scarcity of neurons is characteristic of this layer of the cerebral cortex (Rengifo et al. 2016). Likewise, the intense immunoreactivity to NeuN observed in the pyramidal and granular layers of the hippocampal formation of the mice coincides with the results reported by other authors (Mullen et al. 1992; Bekiari et al. 2020; Collombet et al. 2006). In the cerebellum, exclusive immunoreactivity by the neurons of the inner granular layer to NeuN is highlighted (Weyer and Schilling 2003). Since the discovery of NeuN, it has been known that Purkinje cells are part of the small group of neurons that do not express this protein (Mullen et al. 1992). Weyer and Schilling (2003) conducted a study of the cerebellum of mice of different ages, also using other markers, and demonstrated a lack of NeuN expression in Purkinje cells as well as in neurons of the molecular layer and other interneurons. They also did not find NeuN + cells in the deep cerebellar nuclei or in the outer granular layer. Because NeuN is an exclusive marker of mature or postmitotic neurons (Guselnikova and Korzhevskiy 2015; Sarnat 2015), cells immunoreactive to NeuN are not observed in the subgranular zone of the dentate gyrus nor in the outer granular layer of the cerebellum.
Effect of ZIKV infection on NeuN expression in mouse brain
The intraperitoneal inoculation of ZIKV in neonatal BALB/c mice generated a significant decrease in immunoreactivity to the NeuN protein in different components of the brain. This is evidence of the vulnerability of postmitotic neurons to ZIKV infection. In preliminary tests using RT–PCR, we found a loss of NeuN expression in the cerebral cortex and cerebellum of mice under similar experimental conditions (data not yet published). In a study with the flavivirus of Japanese encephalitis, a loss of immunoreactivity to NeuN was reported in the cerebral cortex of BALB/c suckling mice inoculated intracerebrally (Ghoshal et al. 2007). The authors interpreted the decrease in immunolabeling as neuronal loss in response to the release of inflammatory mediators via microglia activation. In neonatal C57BL/6 mice, another immunocompetent strain, inflammatory events were observed in the brain and cerebellum after being inoculated with ZIKV subcutaneously, but the authors did not report neuronal loss (Ireland et al. 2020).
Neuroinflammation does not necessarily lead to neuronal loss. Human immunodeficiency virus (HIV), without being neurotropic, can affect the CNS through the action of proinflammatory substances such as the HIV-1 Tat protein. HIV infection can reduce the gene and tissue expression of NeuN in some brain areas. A similar effect was observed in a transgenic mouse model that was inoculated with HIV-1 Tat. However, tissue samples processed via the Nissl technique for neuronal counts revealed that there was no neuronal loss (Hahn et al. 2015). There is no doubt that neuronal death is an event inherent to CZS, but infections with ZIKV during advanced pregnancy or in neonates are more likely to leave functional and cognitive sequelae in the nervous system (Lebov et al. 2018; Nielsen-Saines et al. 2019; Ireland et al. 2020). The loss of immunoreactivity to NeuN induced by this viral infection in postnatal mice could reflect neurophysiological alterations. The decrease in NeuN immunoreactivity is more related to a reduction in the intracellular concentration of the protein or loss of its antigenicity (Unal-Cevik et al. 2004; Collombet et al. 2006). The latter seems to be associated with dephosphorylation of this protein (Lind et al. 2005; Maxeiner et al. 2014) .
It has been suggested that the loss of immunoreactivity to NeuN may be a sign of degeneration of differentiated neurons (Collombet et al. 2006; Lavezzi 2015; Yousef et al. 2017). NeuN has been identified as the RNA-binding protein Fox3 (Rbfox3), the antigen recognized by the anti-NeuN antibody (Kim et al. 2009). The Rbfox1, Rbfox2 and Rbfox3 proteins are a family of splicing factors that promote neuronal maturation during development (Jacko et al. 2018). While Rbfox1 and Rbfox2 are also involved in the development of other organs, Rbfox3 is exclusive to neurons (Kim et al. 2013). Rbfox3/NeuN is expressed at the end of neuroblast migration once neurons have established most of their connections and start synthesizing neurotransmitters and the receptors and ion channels in their plasma membrane have been organized. Therefore, the subexpression of Rbfox3/NeuN can be associated with defects in the functional and structural maturation of the nervous system (Sarnat 2015; Jacko et al. 2018; Kim et al. 2013) .
This work provides information to take into consideration when interpreting the pathogenesis of Zika in children who acquired the infection in the second half of pregnancy or in the first postnatal months. Only seven years have passed since the emergence of the Zika pandemic; therefore, some side effects are becoming evident in the child population exposed to the infection but not diagnosed with CZS. The COVID-19 pandemic has interfered with epidemiological follow-up studies on Zika, but new findings and outbreaks associated with ZIKV are expected (Pergolizzi et al. 2021).
References
An H, Cho DW, Lee SE, Yang YS, Han SC, Lee CJ (2016) Differential cellular tropism of lentivirus and adeno-associated virus in the brain of cynomolgus monkey. Exp Neurobiol 25(1):48–54. https://doi.org/10.5607/en.2016.25.1.48
Atapour N, Majka P, Wolkowicz IH, Malamanova D, Worthy KH, Rosa MPG (2019) Neuronal distribution across the cerebral cortex of the marmoset monkey (Callithrix jacchus). Cereb Cortex 29(9):3836–3863. https://doi.org/10.1093/cercor/bhy263
Bekiari C, Grivas I, Tsingotjidou A, Papadopoulos GC (2020) Adult neurogenesis and gliogenesis in the dorsal and ventral canine hippocampus. J Comp Neurol 528(7):1216–1230. https://doi.org/10.1002/cne.24818
Bell TM, Field EJ, Narang HK (1971) Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch 35(2):183–193. https://doi.org/10.1007/BF01249709
Carod-Artal FJ (2016) Epidemiology and neurological complications of infection by the Zika virus: a new emerging neurotropic virus. Rev Neurol 62(7):317–328
Chavali PL, Stojic L, Meredith LW, Joseph N, Nahorski MS, Sanford TJ, Krishna BA, Hosmillo M, Firth AE, Bayliss R (2017) Neurodevelopmental protein Musashi-1 interacts with the Zika genome and promotes viral replication. Science 357(6346):83–88. https://doi.org/10.1126/ciencia.aam9243
Collombet JM, Masqueliez C, Four E, Burckhart MF, Bernabé D, Baubichon D, Lallement G (2006) Early reduction of NeuN antigenicity induced by soman poisoning in mice can be used to predict delayed neuronal degeneration in the hippocampus. Neurosci Lett 398(3):337–342. https://doi.org/10.1016/j.neulet.2006.01.029
Corchuelo S, Gómez CY, Rosales AA, Santamaria G, Rivera JA, Saad EP, Torres-Fernández O, Rengifo AC (2021) CISH and IHC for the simultaneous detection of ZIKV RNA and antigens in formalin-fixed paraffin-embedded cell blocks and tissues. Curr Protoc. https://doi.org/10.1002/cpz1.319
Dick GW (1952) Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 46(5):521–534. https://doi.org/10.1016/0035-9203(52)90043-6
Feuer R, Mena I, Pagarigan RR, Harkins S, Hassett DE, Whitton JL (2003) Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease. Am J Pathol 163(4):1379–1393. https://doi.org/10.1016/S0002-9440(10)63496-7
Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352(6287):816–818. https://doi.org/10.1126/science.aaf6116
Geiselhardt F, Peters M, Kleinschmidt S, Chludzinski E, Stoff M, Ludlow M, Beineke A (2022) Neuropathologic and molecular aspects of a canine distemper epizootic in red foxes in Germany. Sci Rep 12(1):14691. https://doi.org/10.1038/s41598-022-19023-9
Ghoshal A, Das S, Ghosh S, Mishra MK, Sharma V, Koli P (2007) Proinflammatory mediators released by activated microglia induces neuronal death in japanese encephalitis. Glia 55(5):483–496. https://doi.org/10.1002/glia.20474
Gittins R, Harrison PJ (2004) Neuronal density, size, and shape in the human anterior cingulate cortex: a comparison of Nissl and NeuN staining. Brain Res Bull 63(2):155–160. https://doi.org/10.1016/j.brainresbull.2004.02.005
Guselnikova VV, Korzhevskiy DE (2015) NeuN as neuronal nuclear antigen and neuron differentiation marker. Acta Naturae 7(2):42–47
Hahn YK, Masvekar RR, Xu R, Hauser KF, Knapp PE (2015) Chronic HIV-1 Tat and HIV reduce Rbfox3/NeuN: evidence for sex-related effects. Curr HIV Res 13(1):10–20. https://doi.org/10.2174/1570162x13666150311163733
Hight K, Hallett H, Churchill L, De A, Boucher A, Krueger JM (2010) Time of day differences in the number of cytokine-, neurotrophin- and NeuN immunoreactive cells in the rat somatosensory of visual cortex. Brain Res 1337:32–40. https://doi.org/10.1016/j.brainres.2010.04.012
Ireland DDC, Manangeeswaran M, Lewkowicz AP, Engel K, Clark SM, Laniyan A (2020) Long-term persistence of infectious Zika virus: inflammation and behavioral sequela in mice. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1008689
Jacko M, Weyn-Vanhentenryck SM, Smerdon JW, Yan R, Feng H, Williams DJ, Pai J, Xu K, Wichterle H, Zhang C (2018) Rbfox splicing factors promote neuronal maturation and axon initial segment assembly. Neuron 97(4):853–868. https://doi.org/10.1016/j.neuron.2018.01.020
Kim KK, Adelstein RS, Kawamoto S (2009) Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem 284(45):31052–31061. https://doi.org/10.1074/jbc.M109.052969
Kim KK, Nam J, Mukouyama YS, Kawamoto S (2013) Rbfox3-regulated alternativeSplicing of Numb promotes neuronal differentiation during development. J Cell Biol 4:443–458. https://doi.org/10.1083/jcb.201206146
Korzhevskii DE, Gilerovich EG, Zin’kova NN, Grigor’ev IP, Otellin VA (2006) Immunocytochemical detection of brain neurons using the selective marker NeuN. Neurosci Behav Physiol 36(6):857–859. https://doi.org/10.1007/s11055-006-0098-5
Laiton-Donato K, Álvarez-Díaz D, Rengifo AC, Torres-Fernández O, Usme-Ciro JA, Rivera JA, Santamaría G, Naizaque J, Monroy-Gómez J, Sarmiento L, Gunturiz ML, Muñoz A, Vanegas R, Rico A, Pardo L, Peláez-Carvajal D (2019) Complete genome sequence of a colombian Zika virus isolated from BALB/c mouse brain after intraperitoneal inoculation. Microbiol Resour Announc. https://doi.org/10.1128/MRA.01719-18
Lavezzi AM (2015) A new theory to explain the underlying pathogenetic mechanism of sudden infant death syndrome. Front Neurol 6:220. https://doi.org/10.3389/fneur.2015.00220
Lebov J, Brown L, MacDonald P, Robertson K, McCarter-Bowman N, Hooper SR, Becker-Dreps S (2018) Review: evidence of neurological sequelae in children with acquired Zika virus infection. Pediatr Neurol 85:16–20. https://doi.org/10.1016/j.pediatrneurol.2018.03.001
Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z (2016a) Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19(1):120–126. https://doi.org/10.1016/j.stem.2016.04.017
Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, Terskikh AV, Shresta S, Gleeson JG (2016b) Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell 19(5):593–598. https://doi.org/10.1016/j.stem.2016.08.005
Lind D, Franken S, Kappler J, Jankowski J, Schilling K (2005) Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res 79(3):295–302. https://doi.org/10.1002/jnr.20354
Mao Y, Bajinka O, Tang Z, Qiu X, Tan Y (2022) Lung–brain axis: metabolomics and pathological changes in lungs and brain of respiratory syncytial virus-infected mice. J Med Virol 94(12):5885–5893. https://doi.org/10.1002/jmv.28061
Maxeiner S, Glassmann A, Kao HT, Schilling K (2014) The molecular basis of the specificity and cross–reactivity of the NeuN epitope of the neuron–specific splicing regulator, Rbfox3. Histochem Cell Biol 141(1):43–55. https://doi.org/10.1007/s00418-013-1159-9
Mullen R, Buck C, Smith A (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116(1):201–211. https://doi.org/10.1242/dev.116.1.201
Narasimhan H, Chudnovets A, Burd I, Pekosz A, Klein SL (2020) Animal models of congenital Zika syndrome provide mechanistic insight into viral pathogenesis during pregnancy. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0008707
Nielsen-Saines K, Brasil P, Kerin T, Vasconcelos Z, Gabaglia CR, Damasceno L, Pone M, Abreu de Carvalho LM, Pone SM, Zin AA, Tsui I, Salles TRS, da Cunha DC, Costa RP, Malacarne J, Reis AB, Hasue RH, Aizawa CYP, Genovesi FF, Einspieler C, Marschik PB, Pereira JP, Gaw SL, Adachi K, Cherry JD, Xu Z, Cheng G, Moreira ME (2019) Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 25(8):1213–1217. https://doi.org/10.1038/s41591-019-0496-1
Pergolizzi J Jr, LeQuang JA, Umeda-Raffa S, Fleischer C, Pergolizzi J III, Pergolizzi C, Raffa RB (2021) The Zika virus: lurking behind the COVID-19 pandemic? J Clin Pharm Ther 46(2):267–276. https://doi.org/10.1111/jcpt.13310
Piccirilli G, Gabrielli L, Bonasoni MP, Chiereghin A, Turello G, Borgatti EC, Simonazzi G, Felici S, Leone M, Salfi NCM, Santini D, Lazzarotto T (2023) Fetal brain damage in human fetuses with congenital cytomegalovirus infection: histological features and viral tropism. Cell Mol Neurobiol 43(3):1385–1399. https://doi.org/10.1007/s10571-022-01258-9
Rengifo AC, Umbarila VJ, Garzón MJ, Torres-Fernández O (2016) Differential effect of the route of inoculation of rabies virus on NeuN immunoreactivity in the cerebral cortex of mice. Int J Morphol 34(4):1362–1386. https://doi.org/10.4067/S0717-95022016000400031
Sarnat HB (2015) Immunocytochemical markers of neuronal maturation in human diagnostic neuropathology. Cell TissueRes 359(1):279–294. https://doi.org/10.1007/s00441-014-1988-4
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ (2013) Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16. https://doi.org/10.1016/j.pneurobio.2013.04.001
Unal-Cevik I, Kilinç M, Gürsoy-Ozdemir Y, Gurer G, Dalkara T (2004) Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res 1015(1–2):169–174. https://doi.org/10.1016/j.brainres.2004.04.032
Van den Pol AN, Mao G, Yang Y, Ornaghi S, Davis JN (2017) Zika virus targeting in the developing brain. J Neurosci 37(8):2161–2175. https://doi.org/10.1523/JNEUROSCI.3124-16.2017
Wang S, Zhang Q, Tiwari SK, Lichinchi G, Yau EH, Hui H, Li W, Furnari F, Rana TM (2020) Integrin alphavbeta5 internalizes Zika virus during neural stem cells infection and provides a promising target for antiviral therapy. Cell Rep 30(4):969–983. https://doi.org/10.1016/j.celrep.2019.11.020
Wen Z, Song H, Ming GL (2017) How does Zika virus cause microcephaly? Genes Dev 31(9):849–861. https://doi.org/10.1101/gad.298216.117
Weyer A, Schilling K (2003) Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res 73(3):400–409. https://doi.org/10.1002/jnr.10655
Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blümcke I (1996) NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem 44(10):1167–1171. https://doi.org/10.1177/44.10.8813082
Yarandi SS, Robinson JA, Vakili S, Donadoni M, Burdo TH, Sariyer IK (2020) Characterization of Nef expression in different brain regions of SIV-infected macaques. PLoS ONE. https://doi.org/10.1371/journal.pone.0241667
Yousef A, Robinson JL, Irwin DJ, Byrne MD, Kwong LK, Lee EB (2017) Neuron loss and degeneration in the progression of TDP-43 in frontotemporal lobar degeneration. Acta Neuropathol Commun 5(1):68. https://doi.org/10.1186/s40478-017-0471-3
Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, Ji X, Xu YP, Li G, Li C, Wang HJ, Deng YQ, Wu M, Cheng ML, Ye Q, Xie DY, Li XF, Wang X, Shi W, Hu B, Shi PY, Xu Z, Qin CF (2017) A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 358(6365):933–936. https://doi.org/10.1126/science.aam7120
Funding
This work was funded with resources from the Administrative Department of Science, Technology, and Innovation (COLCIENCIAS-MINCIENCIAS) and the Instituto Nacional de Salud (INS-Colombia) (Project Code 210474455818, contract 672 of 2017).
Author information
Authors and Affiliations
Contributions
Conceptualization: ACR, OTF. Data acquisition: GS. Data analysis or interpretation: GS, OT-F. Drafting of the manuscript: OT-F. Critical revision of the manuscript: GS, ACR. Approval of the final version of the manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santamaría, G., Rengifo, A.C. & Torres-Fernández, O. NeuN distribution in brain structures of normal and Zika-infected suckling mice. J Mol Histol 54, 245–253 (2023). https://doi.org/10.1007/s10735-023-10128-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-023-10128-7