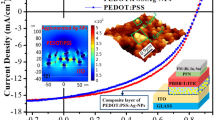
Abstract
Next-generation and solution-processed thin-film solar cells have been attracted considerable attention because of their low cost, light weight, flexibility, and aesthetics. However, most of solution-processed thin-film solar cells are now focused on the use of photovoltaic absorbers containing the toxic element of Pb. In this study, eco-friendly silver-bismuth-iodide (Ag-Bi-I) thin-film photovoltaic devices with high open-circuit voltages (VOC) are developed by utilizing polythieno[3,4-b]-thiophene-co-benzodithiophene (PTB7) as the hole transport layer (HTL). The solution-processed AgBi2I7 semiconductor, which is an Ag-Bi-I ternary compound, exhibit features suitable for photovoltaic layers in thin-film solar cells, including a three-dimensional (3D) crystal structure, good surface morphology, and low optical bandgaps of 1.87 eV. Meanwhile, the solution-processed AgBi2I7 thin-film solar cell based on the PTB7 HTL exhibit a power conversion efficiency of 0.94% with an improved VOC value of 0.71 V owing to the deeper highest occupied molecular orbital (HOMO) energy level compared to that of poly(3-hexylthiophene-2,5-diyl) (P3HT). In other words, the VOC of the PTB7 HTL-based device is 20% higher than that of the P3HT HTL-based control device. Our results provide a new approach for increasing the VOC of eco-friendly Ag-Bi-I thin-film photovoltaics and indicate that further HTL engineering is necessary to simultaneously improve the VOC and performance of the devices.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Solution-processed halide perovskites have emerged as promising photovoltaic absorbers in next-generation thin-film solar cells, with rapid advances in the power conversion efficiencies (PCEs) of perovskite solar cells (PSCs) [1, 2, 3]. Currently, state-of-the-art and high-performance PSCs have achieved PCEs of over 25%, which is close to the theoretical Shockley–Queisser (S–Q) limit efficiency of 30% and exceeds the record PCE of commercialized copper indium gallium selenide (CIGS) thin-film solar cells (23%) [4, 5]. However, previous studies have mostly focused on utilizing lead halide perovskites, which have formidable obstacles that hinder commercialization, such as the toxicity of lead elements and low ambient and thermal stabilities. Therefore, many studies have proposed lead-free and environmentally friendly perovskite-type photovoltaic absorbers consisting of alternative cations, including bivalent Sn2+ and Ge2+ and trivalent Bi3+ and Sb3+ [6, 7, 8, 9, 10].
Among the various candidates, the Sn-based perovskite thin-film solar cells have shown PCEs of over 10%. However, since Sn is also known as a toxic element [11], there are limitations in using Sn-based perovskites as a photovoltaic absorber in eco-friendly thin-film solar cells. Although the current PCE is low, it has been reported that silver-bismuth-iodide (Ag-Bi-I) ternary semiconductors are particularly suitable for commercialization because of their non-toxic elements, suitable electronic configuration, three-dimensional (3D) crystal structure, and high chemical stability [12, 13]. This claim was supported by the recent development of Ag3BiI5.92S0.04, in which sulfur was introduced into Ag3BiI6 for the tuning of optoelectronic properties, which resulted in a record PCE of 5.56% and excellent long-term stability of the Ag-Bi-I-based thin-film solar cell system [14].
Although Ag-Bi-I-based thin-film semiconductors are eco-friendly and lead-free, there are still several issues that need to be resolved. One critical issue is the large energy loss that leads to a low open-circuit voltage (VOC) in Ag-Bi-I-based thin-film solar cells. The VOC of Ag-Bi-I-based thin-film solar cells is significantly low (approximately 0.6 V) [12, 15, 16, 17, 18], especially when considering that the optical bandgap of Ag-Bi-I-based photovoltaic absorbers is approximately 1.8 eV. The energy level offset between the conduction band minimum (CBM) of the TiO2-based electron transport layer (ETL) and the highest occupied molecular orbital (HOMO) energy level of the organic hole transport layer (HTL) [19] significantly affects the VOC. Conventional Ag-Bi-I-based thin-film solar cells reported in the literature employ a device architecture composed of an n-type TiO2 ETL and a p-type organic semiconductor HTL [13]. The CBMs of TiO2 ETLs are similar to those of Ag-Bi-I-based photovoltaic absorbers (~ 4.3 eV) and thus exhibit energy level alignments that are suitable to the extraction of photo-generated charge carriers (i.e., electrons) via the tunneling effect [12].
Furthermore, the HOMO energy levels of HTLs can also have a great effect on improving the photovoltaic performance. 2,2’,7,7’-tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9’-spirobifluorene (spiro-OMeTAD), poly(3-hexylthiophene) (P3HT), and poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA), which exhibit HOMO energy levels of −5.0 ~ −5.1 eV, have been mainly used as HTLs in the most of the solution-processed thin-film solar cells. However, this results in a large energy gap with the valence band maximums (VBMs) of − 6.2 eV for Ag-Bi-I-based photovoltaic absorbers [15, 16, 17]. In this regard, tuning the HOMO energy levels of HTLs close to the VBMs of Ag-Bi-I-based photovoltaic absorbers can potentially further enhance the built-in potential and thus VOC [20]. However, so far there have been no attempts to enhance the VOC of eco-friendly Ag-Bi-I-based thin-film solar cells via HTL engineering.
Herein, we demonstrate the improvement of VOC in eco-friendly and lead-free Ag-Bi-I-based thin-film solar cells by employing polythieno [3,4-b]-thiophene-co-benzodithiophene (PTB7) as the HTL. A solution-processed AgBi2I7 thin-film semiconductor, which is an Ag-Bi-I ternary compound, was utilized as the photovoltaic absorber in the eco-friendly and lead-free thin-film solar cells presented in this study. The AgBi2I7 photovoltaic absorber was fabricated by spin-coating the precursor solution and subsequently annealing it at the desired temperature in an N2-filled glove box. The structural properties, elemental composition, and surface morphology of the solution-processed AgBi2I7 thin-films were also investigated. The PTB7 HTL-based AgBi2I7 thin-film solar cell exhibited an improved VOC of 0.71 V and a similar PCE of 0.94% compared to the P3HT HTL-based control device, which exhibited a VOC of 0.59 V and PCE of 1.02%. The enhanced VOC is attributed to the deeper HOMO energy level of PTB7 compared to that of P3HT, which enhanced the built-in potential. To the best of our knowledge, this is the first demonstration of the utilization of PTB7 as HTLs in eco-friendly Ag-Bi-I-based thin-film solar cells. Furthermore, we highlight that the favorable energy level alignment between the eco-friendly photovoltaic absorbers and the charge transporting layers will greatly contribute to further improving the photovoltaic performance of the lead-free and eco-friendly thin-film solar cells.
2 Results and Discussion
AgBi2I7 thin-films were synthesized and prepared according to the literature [10]. The optical properties, elemental compositions, and crystal structures of the AgBi2I7 thin-films are shown in Fig. 1. The absorption spectrum of the AgBi2I7 thin-film annealed at 150 °C was obtained via UV–Vis spectroscopy (Fig. 1a). The resultant AgBi2I7 thin-film exhibited substantial absorption behavior in the range of 350–750 nm. From the absorption spectrum and corresponding Tauc plot, the optical bandgap (Eg) of the AgBi2I7 thin-film was estimated to be 1.87 eV. X-ray diffraction (XRD) pattern reveals that the crystal structure of the AgBi2I7 thin-film was consistent with that in the literature, and the formation of the cubic-phase was confirmed by the (111), (400), and (440) diffraction peaks at 2θ of 13, 29, and 42°, respectively (Fig. 1b) [12]. From the XRD result, we illustrated the crystal structure of AgBi2I7 using the computer program VESTA (Fig. 1c). The cubic AgBi2I7 unit cell densely and symmetrically consists of a joint population of single positions of Ag, Bi, and I. In the AgBi2I7 unit cell, the Ag and Bi cations coordinate to six and eight I anions, forming octahedral and hexahedral I groups, respectively. Because of the connection between octahedral Ag–I and hexahedral Bi–I from corner-sharing, there were no metal vacancies in the AgBi2I7 crystal structure. Furthermore, to analyze the elemental composition of the AgBi2I7 thin-film, energy-dispersive x-ray spectroscopy (EDX) was utilized as shown in Fig. 1d. The presence of Ag and Bi cations and I anions was verified, and their weight fractions matched the theoretical values well.
a UV–Vis absorption spectrum of the AgBi2I7 thin-films fabricated onto a glass substrate. Inset shows a photographic image of the AgBi2I7 thin-film. b XRD spectrum of the AgBi2I7 thin-films. c The unit cell of an AgBi2I7 crystal. d EDX spectrum of the AgBi2I7 thin-films. Inset shows a table containing the quantitative amounts
The surface morphology of the resultant AgBi2I7 thin-films was characterized using scanning electron microscope (SEM) measurements to examine their potential use as photoactive layers in photovoltaic devices (Fig. 2). Fig. 2a shows the top-view SEM images of the AgBi2I7 thin-films with pinhole-free, dense, and uniform surface morphologies and well-defined boundaries among the grains. The grain sizes were estimated to be 200–800 nm from the high-magnification SEM image in the inset of Fig. 2a. Furthermore, the AgBi2I7 thin-films exhibited flat surface morphologies, as revealed by the cross-sectional SEM image (Fig. 2b). Poor surface morphologies, including pinholes and high surface roughness, can impede the active layer from contact with interlayers and reduce the photovoltaic performance of solar cell devices [21, 22]. However, we confirmed that the AgBi2I7 thin-films are not affected by surface morphological issues and are sufficient to ensure the successful application of Ag-Bi-I thin films in photovoltaic devices.
In addition to photoactive materials, p-type conjugated polymers were investigated for their possible use as HTLs. The chemical structures of P3HT and PTB7 and the energy band diagram of the p-type conjugated polymers and AgBi2I7 thin-films are shown in Fig. 3a and 3b, respectively. The energy level values for AgBi2I7, P3HT, and PTB7 are from previous studies [12, 23]. PTB7, a representative donor–acceptor (D–A) type low bandgap polymer [24], possesses deeper LUMO (−3.6 eV), HOMO (−5.2 eV), and Fermi energy levels than P3HT (LUMO = −3.0 eV; HOMO = −5.0 eV). If a PTB7 HTL is introduced into AgBi2I7 thin-film solar cells, the underlying HOMO energy level of PTB7 can enhance the built-in potential, resulting in a VOC improvement. Moreover, a low energy offset with the VBM of AgBi2I7 facilitates hole extraction from the photoactive layer to the HTL. Additionally, it is well known that PTB7 backbones prefer a face-on orientation relative to the substrate, which is beneficial for hole mobility and charge transport through the HTL to the top electrode [24, 25, 26, 27, 28].
To elucidate the effect of the PTB7 polymer on bismuth-based solar cell systems, we fabricated photovoltaic devices using conjugated polymers as HTLs with a device architecture of fluorine-doped tin oxide (FTO)/compact or mesoporous TiO2 (c-TiO2 or m-TiO2)/AgBi2I7/P3HT or PTB7/Au (Insets of Fig. 4 and Fig. S1). The cross-sectional SEM images of the devices in Fig. 4 present that there was no significant difference between the morphologies of P3HT and PTB7 HTLs on the AgBi2I7 photovoltaic absorbers. Thus, there was no the effect of the film morphologies on the device properties such as charge extraction. Fig. 4 and Fig. S2 show the current density versus voltage (J–V) characteristics of the optimized solar cell devices measured under AM 1.5 G simulated solar illumination (100 mW cm−2), and Table 1 summarizes the corresponding photovoltaic characteristics. The best PCEs of the P3HT-based devices with c-TiO2 and m-TiO2 were 0.53% (VOC = 0.59 V; short-circuit current density (JSC) = 1.59 mA cm−2; fill factor (FF) = 55.7%) and 1.02% (VOC = 0.56 V; JSC = 3.46 mA cm−2; FF = 52.1%) as a reverse scan. The enhanced efficiency and current density originated from the increased contact area between the ETL and active layer owing to the mesoporous morphology of TiO2. Based on these results, we also adopted m-TiO2 as ETLs in the PTB7 solar cell systems. Compared with the reference device, the VOC of the PTB7-based device improved remarkably by 20%, from 0.59 to 0.71 V, with a JSC of 2.41 mA cm−2, an FF of 55.1%, and an efficiency of 0.94%. The higher VOC is attributed to the deeper HOMO energy level of PTB7. The enhanced FF resulted from the better energy-level alignment and charge-transport ability of the PTB7 HTL. The decrease in the current density could be attributed to the low LUMO energy level of PTB7, which is slightly different from the CBM of AgBi2I7 (−4.3 eV) and allows electrons to be transported through the AgBi2I7 thin-film to the HTL [29, 30]. Furthermore, the electron leakage could lead to the hysteresis identified from the J–V curve of the PTB7-based device as shown in Fig. 4b. Hysteresis can be originated from ferroelectric effect, trap-assisted charge recombination, and ion migration in perovskite materials, and unbalanced charge transport/extraction in photovoltaic devices [31]. In the case of the PTB7 systems, electron leakages from the AgBi2I7 thin-film to the HTL due to the low LUMO energy level of PTB7 could cause unbalanced charge extraction, resulting in the hysteresis of the PTB7-based photovoltaics.
3 Conclusion
In summary, we developed AgBi2I7 thin-film-based solar cell devices with high open-circuit voltages by introducing PTB7 as the HTL. The PCE (0.94%) was similar to that of previous bismuth-based solar cell devices, and was attributed to the dense and pinhole-free surface morphologies and the AgBi2I7 thin-film’s relatively suitable bandgap (1.87 eV). The improved VOC (0.71 V) was attributed to the lower HOMO energy level of PTB7 compared to that of P3HT. The utilization of PTB7 as HTLs in Ag-Bi-I-based solar cells was demonstrated for the first time. Furthermore, the results from the photovoltaic devices indicate that the energy level alignment and interface charge extraction between the AgBi2I7 photoactive layers and interlayers are significant and require further research. If hole transporting polymers, which have good hole mobilities and higher LUMO and lower HOMO energy levels than PTB7, are introduced as a HTL in the photovoltaic devices, both VOC and PCE can be enhanced simultaneously.
4 Materials and Methods
4.1 Materials
Bismuth iodide (BiI3), silver iodide (AgI), n-butylamine (BTA), titanium isopropoxide (TTIP), hydrochloric acid (HCl), and other organic solvents were purchased from Sigma-Aldrich. Poly(3-hexylthiophene-2,5-diyl) (P3HT) and polythieno[3,4-b]-thiophene-co-benzodithiophene (PTB7) were purchased from 1-Material.
4.2 AgBi2I7 Film Fabrication
0.30 g of BiI3 and 0.06 g of AgI were completely dissolved in 3.0 mL of BTA by stirring for 1 h. Oxygen (O2) plasma treatment was performed for substrate cleaning and obtaining a hydrophilic surface before spin-coating. The precursor solution was spin-cast at 6000 rpm for 30 s onto the O2 plasma-treated substrates under a humidity below 20%, and then the film was immediately transferred to a N2-filled glove box to be ready for thermal annealing. The resulting film was heated to 150 °C at a constant rate of 5 °C min−1 and maintained at that temperature for 30 min. Next, the resulting film was quenched to room temperature and exhibited a brown-black color.
4.3 Device Fabrication
AgBi2I7 films were prepared on both compact and mesoporous TiO2. The patterned FTO substrates were cleaned via sequential sonication in acetone, isopropyl alcohol, and deionized water for 20 min each. 0.74 mL of TTIP was slowly added to 8 mL of anhydrous ethanol under vigorous stirring, and 0.06 mL of HCl was rapidly injected into the solution to prepare the precursor for the compact TiO2 layer. The resulting solution was then stirred overnight without heating. The TiO2 precursor was first spin-cast at 2000 rpm for 30 s onto the FTO substrates, and the resulting substrates were then thermally annealed at 500 °C for 60 min. After cooling to room temperature, the substrates were soaked in a low-concentration TiCl4 aqueous solution (120 mM, 70 °C) for 30 min and then annealed at 500 °C for 60 min to improve the interfacial properties of the compact TiO2 layer. For the mesoporous TiO2 structure, diluted TiO2 paste (DS18) in ethanol was spin-cast at 5000 rpm for 60 s onto compact TiO2-coated FTO substrates. The substrates were thermally annealed at 500 °C for 60 min, followed by TiCl4 treatment and annealing as described above. After coating the AgBi2I7 film, P3HT and PTB7 in chlorobenzene (10 mg mL‒1) were spin-coated as hole-transporting materials (HTMs) at 4000 rpm for 30 s and annealed at 120 °C for 30 min. Finally, patterned gold electrodes were deposited as top metal contacts using a thermal evaporator with a shadow mask.
4.4 Device Measurement
A Keithley 2400 Source Meter was used to measure current and voltage. The solar spectrum at AM 1.5 was simulated to within class A specifications (< 25% spectral mismatch) using a Xe lamp and filters (Solar Light Company Inc.) with a measured intensity of 100 mW cm−2. The illumination intensity was calibrated using a Melles–Griot broadband power meter and Thorlabs broadband power meter via a circular optical aperture (area 0.049 cm2) at the position of the device and verified with a calibrated reference Si solar cell (Newport, Inc.). The final accuracy of the solar-to-electricity measurements was approximately ± 5%.
4.5 Characterization Methods
Bruker D2 Phaser X-ray diffractometer with Cu Kα radiation (λ = 1.5406 Å) was used to preform X-ray diffraction (XRD) measurements. A field-emission scanning electron microscope (FE-SEM, Hitachi SU8230) was used to obtain top-view surface morphological images and energy-dispersive X-ray spectroscopy (EDX) spectra. A Shimadzu UV-2600 UV–vis–NIR spectrophotometer was utilized to obtain absorption spectra.
References
Kojima, A., Teshima, K., Shirai, Y., Miyasaka, T.: Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009)
Burschka, J., Pellet, N., Moon, S.-J., Humphry-Baker, R., Gao, P., Nazeeruddin, M.K., Grätzel, M.: Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316–319 (2013)
Yang, W.S., Noh, J.H., Jeon, N.J., Kim, Y.C., Ryu, S., Seo, J., Seok, S.I.: High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348, 1234–1237 (2015)
NREL Best research-cell efficiency chart. https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf. Accessed 30 Mar. 2023
Park, N.-G., Segawa, H.: Research direction toward theoretical efficiency in perovskite solar cells. ACS Photonics 5, 2970–2977 (2018)
Yang, X., Chen, Y., Liu, P., Xiang, H., Wang, W., Ran, R., Zhou, W., Shao, Z.: Simultaneous power conversion efficiency and stability enhancement of Cs2AgBiBr6 lead-free inorganic perovskite solar cell through adopting a multifunctional dye Interlayer. Adv. Funct. Mater. 30, 2001557 (2020)
Ke, W., Kanatzidis, M.G.: Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 10, 965 (2019)
Fan, Q., Biesold-McGee, G.V., Ma, J., Xu, Q., Pan, S., Peng, J., Lin, Z.: Lead-free halide perovskite nanocrystals: crystal structures, synthesis, stabilities, and optical properties. Angew. Chem.-Int. Edit. 59, 1030–1046 (2020)
Xiao, Z., Song, Z., Yan, Y.: From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives. Adv. Mater. 31, 1803792 (2019)
He, Z., Yu, R., Song, W., Gong, Y., Li, H., Tan, Z.A.: Bismuth complex controlled morphology evolution and CuSCN-induced transport improvement enable efficient BiI3 solar cells. Nanomaterials 12, 3121 (2022)
Babayigit, A., Ethirajan, A., Muller, M., Conings, B.: Toxicity of organometal halide perovskite solar cells. Nat. Mater. 15, 247–251 (2016)
Kim, Y., Yang, Z., Jain, A., Voznyy, O., Kim, G.-H., Liu, M., Quan, L.N., García de Arquer, F.P., Comin, R., Fan, J.Z., et al.: Pure cubic-phase hybrid iodobismuthates AgBi2I7 for thin-film photovoltaics. Angew. Chem.-Int. Edit. 55, 9586–9590 (2016)
He, Z., Gong, Y., Tan, Z.A.: Recent advances in bismuth-based solar cells: fundamentals, fabrication, and optimization strategies. Adv. Sustain. Syst. 6, 2200051 (2022)
Pai, N., Lu, J., Gengenbach, T.R., Seeber, A., Chesman, A.S.R., Jiang, L., Senevirathna, D.C., Andrews, P.C., Bach, U., Cheng, Y.-B., et al.: Silver bismuth sulfoiodide solar cells: Tuning optoelectronic properties by sulfide modification for enhanced photovoltaic performance. Adv. Energy Mater. 9, 1803396 (2019)
Jung, K.W., Sohn, M.R., Lee, H.M., Yang, I.S., Sung, S.D., Kim, J., Wei-Guang Diau, E., Lee, W.I.: Silver bismuth iodides in various compositions as potential Pb-free light absorbers for hybrid solar cells. Sustain. Energ. Fuels 2, 294–302 (2018)
Zhu, H., Pan, M., Johansson, M.B., Johansson, E.M.J.: High photon-to-current conversion in solar cells based on light-absorbing silver bismuth iodide. Chemsuschem 10, 2592–2596 (2017)
Lu, C., Zhang, J., Sun, H., Hou, D., Gan, X., Shang, M.-H., Li, Y., Hu, Z., Zhu, Y., Han, L.: Inorganic and lead-free AgBiI4 rudorffite for stable solar cell applications. ACS Appl. Energy Mater. 1, 4485–4492 (2018)
Kulkarni, A., Jena, A.K., Ikegami, M., Miyasaka, T.: Performance enhancement of AgBi2I7 solar cells by modulating a solvent-mediated adduct and tuning remnant BiI3 in one-step crystallization. Chem. Commun. 55, 4031–4034 (2019)
Guo, Z., Jena, A.K., Kim, G.M., Miyasaka, T.: The high open-circuit voltage of perovskite solar cells: a review. Energy Environ. Sci. 15, 3171–3222 (2022)
Kim, Y., Jung, E.H., Kim, G., Kim, D., Kim, B.J., Seo, J.: Sequentially fluorinated PTAA polymers for enhancing VOC of high-performance perovskite solar cells. Adv. Energy Mater. 8, 1801668 (2018)
Park, B.-W., Philippe, B., Zhang, X., Rensmo, H., Boschloo, G., Johansson, E.M.J.: Bismuth based hybrid perovskites A3Bi2I9 (A: Methylammonium or cesium) for solar cell application. Adv. Mater. 27, 6806–6813 (2015)
Lyu, M., Yun, J.-H., Cai, M., Jiao, Y., Bernhardt, P.V., Zhang, M., Wang, Q., Du, A., Wang, H., Liu, G., et al.: Organic–inorganic bismuth (III)-based material: a lead-free, air-stable and solution-processable light-absorber beyond organolead perovskites. Nano Res. 9, 692–702 (2016)
Yao, H., Zhang, H., Ye, L., Zhao, W., Zhang, S., Hou, J.: Molecular design and application of a photovoltaic polymer with improved optical properties and molecular energy levels. Macromolecules 48, 3493–3499 (2015)
Liang, Y., Xu, Z., Xia, J., Tsai, S.-T., Wu, Y., Li, G., Ray, C., Yu, L.: For the bright Future—bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 22, E135–E138 (2010)
Du, Y., Cai, H., Ni, J., Li, J., Yu, H., Sun, X., Wu, Y., Wen, H., Zhang, J.: Air-processed, efficient CH3NH3PbI3−xClx perovskite solar cells with organic polymer PTB7 as a hole-transport layer. RSC Adv. 5, 66981–66987 (2015)
Aqoma, H., Mubarok, M.A., Lee, W., Hadmojo, W.T., Park, C., Ahn, T.K., Ryu, D.Y., Jang, S.-Y.: Improved processability and efficiency of colloidal quantum dot solar cells based on organic hole transport layers. Adv. Energy Mater. 8, 1800572 (2018)
Yuan, J., Ling, X., Yang, D., Li, F., Zhou, S., Shi, J., Qian, Y., Hu, J., Sun, Y., Yang, Y., et al.: Band-aligned polymeric hole transport materials for extremely low energy loss α-CsPbI3 perovskite nanocrystal solar cells. Joule 2, 2450–2463 (2018)
Jeong, I., Jo, J.W., Bae, S., Son, H.J., Ko, M.J.: A fluorinated polythiophene hole-transport material for efficient and stable perovskite solar cells. Dyes Pigment. 164, 1–6 (2019)
Arumugam, G.M., Karunakaran, S.K., Liu, C., Zhang, C., Guo, F., Wu, S., Mai, Y.: Inorganic hole transport layers in inverted perovskite solar cells: a review. Nano Select 2, 1081–1116 (2021)
Yang, G., Zhu, Y., Huang, J., Xu, X., Cui, S., Lu, Z.: Hole transport layer selection toward efficient colloidal PbS quantum dot solar cells. Opt. Express 27, A1338–A1349 (2019)
Singh, R., Parashar, M.: Origin of Hysteresis in Perovskite Solar Cells. In: Ren, J., Kan, Z. (eds.) Soft-Matter Thin Film Solar Cells, pp. 1–42. AIP Publishing, New York (2020)
Acknowledgements
This research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (NRF-2021M3H4A3A01062966).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwon, TG., Kim, T. & Kim, Y. Enhanced Open-Circuit Voltage of Eco-Friendly Silver Bismuth Iodide Thin-Film Photovoltaics with PTB7 Polymer-Based Hole Transport Layer. Electron. Mater. Lett. 20, 165–172 (2024). https://doi.org/10.1007/s13391-023-00437-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-023-00437-0