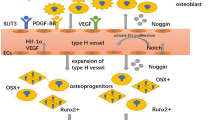
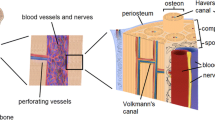
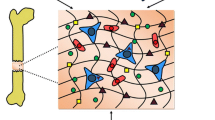
Abstract
The revascularization of grafted tissues is a complicated and non-straightforward process, which makes it challenging to perform reconstructive surgery for critical-sized bone defects. This challenge is combined with the low vascularity that results from radiotherapy. This low vascularity could result from ischemia–reperfusion injuries, also known as ischemia which may happen upon grafting. Ischemia may affect the hard tissue during reconstruction, and this can often cause resorption, infections, disfigurement, and malunion. This paper therefore reviews the clinical and experimental application of procedures that were employed to improve the reconstructive surgery process, which would ensure that the vascularity of the tissue is maintained or enhanced. It also presents the key strategies that are implemented to perform tissue engineering within the grafted sites aiming to optimize the microenvironment and to enhance the overall process of neovascularization and angiogenesis. This review reveals that the current strategies, according to the literature, are the seeding of the mature and progenitor cells, use of extracellular matrix (ECM), co-culturing of osteoblasts with the ECM, growth factors and the use of microcapillaries incorporated into the scaffold design. However, due to the unstable and regression-prone capillary structures in bone constructs, further research focusing on creating long-lasting and stable blood vessels is required.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Reconstructive surgery for critical-sized defects is still considered a challenge due to poor tissue perfusion, called ischemic–reperfusion injury or ischemia [1]. Bone healing can still occur with the initial transient decrease in blood flow, and angiogenesis and revascularization are necessary for normal bone healing [2]. Tissue necrosis of the flap can affect hard tissue-like bone during reconstruction, resulting in infection, resorption, malunion, and disfigurement.
Angiogenesis has been defined as the formation of new vessels from pre-existing vascular networks through the proliferation and migration of differentiated endothelial cells [3]. Whereas neovascularization occurs in the case of inflammation, malignancy, and ischemia [4]. Neovascularization was defined in more detail by Velazque, who stated that local factors at the site of inflammation or tissue injuries stimulate angiogenesis from the existing vasculature and promote the recruitment of circulating bone marrow-derived endothelial precursor cells (EPCs) that contribute to vasculogenesis [5].
Physiologic angiogenesis passes through three stages: stimulus, initiation, and resolution. The stimulus can trigger an angiogenesis cascade. In the initiation phase, an increase in endothelial cell-derived growth factors (GFs), such as vascular endothelial growth factor A (VEGFA) and fibroblast growth factor (FGF), cause vessel destabilization and initiate vessel sprouting and endothelial cell proliferation. Matrix metalloproteinases (MMPs) facilitate the remodeling of the extracellular matrix (ECM) and increase the biological availability of ECM-sequestered growth factors. Finally, the resolution phase starts once the new vessels are formed and perfusion is established in which VEGF levels decline with a rise in platelet-derived growth factor (PDGF), angiopoietins (Ang), and transforming growth factor-β1 (TGFβ1) (Chung et al. 2010) [6]
The process of neovascularization in adult involve manly angiogenesis and percentage of vasculogenesis (3.5% and 25%) in a single microenvironment [7, 8]. The process of neovascularization starts under the influence of number of factors including hypoxia, hypoglycemia, mechanical stresses, inflammation and genetic mutations related to cancer or disease.
In General, it stimulates migration of Vascular Endothelial cells (VEC) into extracellular matrix, then assembling network structures. The role of other supporting cells like pericytes, vascular mural cells are also important in keeping vessel stability, mechanical function and response to physiological changes.
In bone regeneration two biological mechanisms were proposed in literature, for direct bone healing, VEC and perivascular mesenchymal cells provide the osteoprogenitor cells that differentiate into osteoblasts and create lamellar bone to achieve complete union during direct fracture healing. On other hand, vascular endothelial growth factor (VEGF) is considered a key regulator of vascular regeneration in endochondral fracture healing. More vascularization and blood perfusion related to the greater inflammatory response and higher angiogenic cytokine secretion in case of indirect fractur healing may occur [9].
Neovascularization, and osteogenesis are integral to acute fracture healing and have been an important component of studies in bone tissue engineering [10], which has been defined as “The application of the principle and methods of engineering and the life sciences toward the development of biological substitution to restore, maintain or improve functions” [11]. Cultivating vascularity and inducing robust neovascularization is the key of success in reconstructive surgery. Cell-based therapy using biomimetic scaffolds that provide microenvironmental and inductive extra-cellular cues and that can preserve host cell properties when implanted in vivo are necessary for optimum tissue engineering.
Different methods have been proposed in the literature to overcome the problem of ischemic reperfusion injuries at the clinical and pre-clinical levels and were included in this review (see Fig. 1). This review discusses previous and current trends regarding the induction of angiogenesis and neovascularization using tissue engineering at the clinical and ex vivo levels.
Schematic illustration summarizing the three main strategies to stimulate neovascularization and angiogenesis to overcome the problem of ischemia while grafting. 1. Using cell-based therapy to sustain the angiogenic process through the secretion of vascular growth and inhibition of proinflammatory mediators. 2. Inhibition of proinflammatory mediators can occur through the use of different bioactive scaffolds and the use of biomimetic scaffolds. 3. The use of medications and mechanical and physico-chemical stimuli were proven to stimulate and sustain the process of neovascularization or to prevent ischemic perfusion injury
2 Methodology
Search methodology: This research was based on the Population Intervention Comparison Outcome Study design (PICOS) format. The PubMed, Web of Science, Cochrane, and Ovid databases were used for the literature search. The inclusion and exclusion criteria are listed in Table 1. Clinical human trials, case series, and case reports that investigated the role of tissue engineering to overcome the problem of vascularity in repairing critical-sized bone defects using cell-based therapy, bone morphogenic proteins, or physiochemical stimulation. Summary for the selected papers based on the inclusion criteria and the strategy used to induce neo-vascularization are highlighted (Table 2).
3 Which patients require bone/tissue engineering?
There are particular patient categories that require tissue and bone engineering because of the nature of their medical and health conditions. The first category of patients among these are patients who experience chronically upregulated immune systems. These conditions are experienced in patients such as those with rheumatoid arthritis, systemic lupus erythematosus, or diabetes. Another group is patients who have experienced acute dysregulation of their immune systems. One example of this can be seen in polytrauma and sepsis patients. Some of the risk factors for nonunion include smoking, patients with old age, diabetic patients, and patients using non-steroidal anti-inflammatory medications [12, 13].
4 Literature-reported methods of avoiding ischemia–reperfusion injuries
4.1 Outline of the clinical and experimental works for the induction of angiogenesis and revascularization by utilizing skeletal muscles/active biomaterial
Evidence in the literature shows that when muscles are exposed to osteogenic stimuli, such as bone matrix substitutes, or to Bone morphogenic proteins, they develop some intrinsic osteogenic properties that are crucial in developing and facilitate the process of bone formation [14,15,16,17,18]. Previous studies have demonstrated the colonization of the endothelial cells into muscle tissues when used as scaffolds make it possible to reestablished a three-dimensional vascular network after the implantation process [19]. The aim of the clinical case reports and ex vivo experiments was to target the use of the skeletal muscles as a bioreactor. These case studies have been discussed [15, 16, 18,19,20] in vivo experiments. For these reasons, most of the case studies have suggested the use of the skeletal muscles as a biomimetic supporting scaffold in order to facilitate the bone formation process, which also provides the much-needed vascularity. Summary for the role of biomimetic scaffolds is critical in the maintenance of stem cell properties and the possibility of reprogramming their commitment. They support and maintain either a differentiated or an undifferentiated status of the mesenchymal stromal cells (MSCs). There are different elements that could affect the cells; these are oxygen tension, glucose concentration, growth factors, and the physiochemical nature of the environment including the pH and ionic strength (e.g., Ca2+ concentration) [21]. In addition, the use of biomimetic scaffolds was proven to allow the modulation of the immune environment in favor of regenerative processes and suppression of tissue degeneration [22].
The clinical case reported by Heilotis et al. included a case of a 60-year-old patient who was awaiting hemimandibulectomy following radiotherapy. The procedure involved the adjustment of the three blocks of hydroxyapatite HA to resemble the shape of the ramus and mandibular body onto Pectoralis muscle for a 15-weeks period. These costructs were infused with BMP-7, then implemented within the mandibular defect. The patient later developed the MRASA [16].
The study by Warnke et al. provided the case of a clinical case study in which rhBMP-7 was seeded with mesenchymal stromal cells (BMSCs). These were then used together with a titanium mesh tray to establish an external scaffold and were added to a degradable polylactide scaffold. In this report, the prepared construct was implanted into the latissimus dorsi muscle of the patient over a period of 7 weeks. However, in this case, the patient died from cardiac arrest within 15 months of the implantation [15].
Further clinical experimentations suggest the use of In vivo osteogenic properties for the grafting of skeletal muscles. Such experimentation shows that novel strategies can be implemented to facilitate effective bone regeneration [19, 20, 23]. For example, more recently, it has been established that decellularized skeletal muscles (Fig. 2) can be utilized for bone augmentation to facilitate regeneration and to restore the contour and volume of the lost bone. The experiment also included histological assessment that showed the formation of neovascularization which showed a potential graft strategy for patient presented with massive bone resorption or critical-sized defects [24, 25].
4.2 Jaw reconstruction using bone morphogenic proteins (BMPs), growth factors, and scaffolds
BMPs are one of the vital elements of the vascular system. This vitality was previously established in experimental studies that involved both rat models and human vascular disorders, as shown by ref. [26]. BMPs play crucial roles in the process of endothelial cell differentiation, as well as during the process of venous/arterial and lymphatic specification. Goumans et al., in their experiment, demonstrated clearly that the signaling process using bone morphogenetic proteins plays a critical role in regulating vascular functioning [26].
Additionally, the role of bioactive third-generation scaffolds was also highlighted in the literature. Zhai et al. reported that silica (Si) ions that are present in active bioceramics, such as silica calcium phosphate cement (SCPC), stimulated the human umbilical vein endothelial cells to highly express vascular endothelial growth factors (VEGFs) and subsequently activated kinase domain insert receptors by themselves to initiate the angiogenesis pathway [27]. Similarly, Deng et al. reported the addition of interferon-gamma I to calcium silica oxide (CaSiO3) added to beta tricalcium phosphate (β-TCP) 3D-printed scaffolds, indicating the effectiveness of the dual release of IFN-γ and Si from scaffolds in modulating macrophage polarization and promoting vascular ingrowth [28].
Various emerging strategies by which to enhance vascularization in bone grafts have been proposed in the literature using three-dimensional (3D) printed scaffolds. Some used a simple implantation of growth factors, which is known to enhance vascularization, while others were more sophisticated, using fabricated vasculature inside the 3D scaffolds in vitro, which were then directly anastomosed or micro-surgically connected once implanted in vivo [29]. The following are examples of 3D-treated scaffolds for bone regeneration: VEGF was conjugated into 3D-fabricated polycaprolactone (PCL) and hydroxyapatite (HA), resulting in a 3D-printed scaffold PCL/HA with interconnected hollow-pipe structures [30, 31]. In 2019, Yan reported the use of deferoxamine (DFO)-loaded PCL scaffolds. Vascularized 3D-printed scaffolds have been used for the promotion of bone regeneration [32]. Additionally, angiogenic peptide and osteogenic peptide were loaded into tricalcium phosphate/polylactic glycolic acid (TCP/PLGA) composite scaffolds were constructed via cryogenic 3D printing and subsequent hydrogel coating [33]. Moreover, a 3D-printed scaffold was made of a highly elastic polycaprolactone (L-lactide-co-ε-caprolactone) (PLCL), and a hydrogel was made with decellularized adipose tissue extracellular matrix (ECM) and collagen. The 3D-printed scaffolds were subcutaneously implanted in rats for in vivo experiments [34]. The latter reported a higher vascularization rate when compared with the use of PLCL for bone defects in an animal model.
Finally, artificial microvessels were implemented inside a 3D-printed scaffold, which was implanted in vivo [35, 36]. Another example is the model constructed by Zhang et al., which was made of silicate bioceramic scaffolds with hollow struts, and a successful result was reported [36].
Clinically, Moghadam et al. studied the use of allogeneic bone, which was infused with BMP together with their reconstruction plate. This was implemented in one patient in whom the ameloblastoma of the mandible was also removed [37]. In the study by Ferreti and Ripamotnit, 13 patients were involved in an experiment in which collagen was used as carrier for BMP2, aiming at solving the challenge of mandibular continuity defect reconstruction [38]. In the clinical by Marx et al. 40 patients were involved who require mandibular reconstruction. A composite grafts was made using rhBMP-2 added to crushed cancellous freeze-dried allogeneic bone (CCFDAB), and platelet-rich plasma (PRP) was used for experimental group, whereas the control group was grafted using autogenous graft. All the successful autogenous grafts regenerated sufficient bone for implant restoration compared to 97.4% of the composite grafts [39].
The study by Chao et al. also reported a case of a 12 cm ossifying fibroma reported in a 9-year-old male patient. These cases were handled using 36-weeks intervention plan that involved the use of a reconstruction plate, a collagen carrier for rhBMP-2 and stem cell concentration, which were all included in the Tricalcium phosphate/hydroxyapatitte (TCP + HA) [40]. A further study by Herford et al. later reported an experiment that showed successful reconstruction. This process was successfully undertaken among 12 patients using type I collagen and the BMP-2 [41].
Cicciu et al. also studied the case of reconstruction within a patient who experienced an en-bloc resection in the dentinogenic ghost cell. In this process, the medical practitioners used an inferior titanium plate, and then added rhBMP-2 to the Collagen Sponge with the help of an allograft. After a period of 9 months, they then removed the titanium mesh, and they were able to insert dental implants in the patient [42]. An additional study conducted by Ayoub et al. also reported the reconstruction of an alveolar cleft using 3.5 mg of the recombinant human bone morphogenic protein-7 (rhBMP-7). This study, which involved at least 11 patients, utilized the type I collagen carrier and similarly presented promising results regarding the use of this reconstruction method [43].
Despite the existence of the many success stories that have led to significant milestones in reconstruction, it is important to note that, in the cases where only BMP was used, the studies reported a 13.9% rate of failure. This is especially significant when reconstruction is carried out involving large bone defects and defects located mainly in the maxillofacial region of the human body [44].
4.3 Mesenchymal stromal cell (MSC)-based therapy
The rationale for using cell-based therapy is based on two main factors: in addition to providing a multipotency of the cell to transform into a different cell language, cell therapy also presents the opportunity for cells to stimulate or suppress the immune system. The suppression of the enhancement of the immune system follows the varied intervention of macrophages, T and B cells, dendritic cells, and natural killer cells. Additionally, MSCs may be considered therapeutic agents in inflammation treatment (Jin 2022) [45]. On other hand, stromal cells are capable of sustaining the angiogenic process through the secretion of vascular growth- and inflammation-promoting factors, granulocyte colony-stimulating factors (GCSF), and interleukin1β (IL-1β,) resulting in a failure to resolve the angiogenesis cascade [6].
Understanding the impacts of cell theory is vital for the treatment of certain conditions, such as patients who have experienced acute dysregulation of their immune systems. Such dysregulation is normally seen in sepsis or polytrauma cases[46]. Other cases are also experienced by patients with systemic lupus erythematosus, rheumatoid arthritis, or diabetes [46]. Several researchers have also reported the significance of employing cell therapy in such processes, especially for neovascularization and angiogenesis. This takes place after modifying, pre-conditioning, and even genetically inducing the seeded cell [47, 48].
Several clinical report cases have also been conducted utilizing cell-based therapy. The first of these was the report by Kioshita et al. This study used A-poly L-lactic acide PLLA mesh, which was designed in the shape of an alveolar bridge. In this case, the mesh was used to reconstruct the mandible areas with the help of the autogenous particulate cancellous bone together with the whole bone marrow aspirate. The cells were left intact without separation. In two of the clinical cases, the augmentation was carried out through the use of hydroxyapatite. Overall, the clinical report showed significantly positive outcomes for reconstruction defects in the mandibles [49].
The second case report in this category was conducted by Lee et al., and this report involved the case of a single patient in whom the resected jaw was frozen then the reconstruction was made using differentiated MSCS and fibrin glue. Guided bone regeneration was used to guide the overall process of the bone generation techniques. Subsequently, the report was concluded with the use of a dental implant to replace the dental structure [50]. A similar technique was also studied in each clinical case report by Hernadnems-Afaro et al. In this case, the report involved a 33-year-old patient who presented with a complication of recurrent ameloblastoma, and with a titanium mesh. In this case, the patient was infused with 2 g of rBMP-7 and 5 mL of bone marrow concentrate. After nine months, a surgical re-entry was performed for an implant placement [51].
In the study by Zamiri et al., three serious cases were reported in which the patients all had mandibular defects of a critical size. In these cases, autogenous MSCs and mandibular bone were implemented to perform the reconstruction. Among these cases, one reported failure, while the others reported success [52]. In a similar study, the case discussed by Kim et al. involved mandibular reconstruction, which was conducted using autologous human bone marrow stem cells (BMSCs). This process was combined with autogenous bone grafting to restore mandibular defect in patient with plexiform ameloblastoma. The grafting was performed for this patient 6 months after the initial operations, and this also allowed planning for the implant. The report, therefore, stated that the use of BMSCs is a viable alternative to the previous method of autologous bone grafting [53].
Another case study was also presented, in which three patients were grafted using adipose stem cells (ASCs) seeded into a β-TCP scaffold. As reported by Wolff et al., these stem cells were then infused with the BMP-2 [54]. During the same period, a study by Sandor et al. also reported a case of successful reconstruction that was conducted using a reconstruction plate with ASCs/β-TCP and BMP-2. Ten months after the reconstruction surgery, six endosseous dental implants were inserted in the regenerated bone for dental rehabilitation [55].
5 Conclusions
This review illustrates different strategies by which to overcome the problem of ischemic–reperfusion injury upon grafting critical-sized bone defects. The current trends described in the literature are the seeding of the mature and progenitor cells into extracellular matrix (ECM), co-culturing of osteoblasts with the ECM, growth factors, and the use of microcapillaries into scaffold design. However, due to the unstable and regression-prone capillary structures in bone constructs, further research focusing on creating long-lasting and stable blood vessels and the incorporation of vasculature into individual compartments is required.
5.1 Data sharing
Not applicable to this article as no datasets were generated or analyzed during the current study
Abbreviations
- EC:
-
extracellular
- ECM:
-
extra cellular matrix
- GF:
-
growth factors
- VEGFA:
-
vascular endothelial growth factors
- FGF:
-
fibroblast growth factor
- MMP:
-
Matrix metalloproteinases
- PDGF:
-
platelet-derived growth factor
- Ang:
-
angiopoietins
- FGEß1:
-
transforming growth factor beta-1
- MSCs:
-
mesenchymal stromal cells
- BMP:
-
bone morphogenic protein
- SCPC:
-
silica calcium phosphate cement
- ß-TCP:
-
beta tricalcium phosphate
- CaSiO:
-
calcium silica oxide
- 3D:
-
three-dimensional
- PCL:
-
poly caprolactone
- DFO:
-
deferoxamine
- HA:
-
hydroxyapatite
- PLCL:
-
L-LACTIDE-CO-Œ-Ca prolactone
- rhBMP-7:
-
recombinant human bone morphogenic protein
- GCSF:
-
granulocyte colony-stimulating factor
- IL-1ß:
-
interleukin 1 beta
- VEC:
-
vascular endothelial cells
References
Kang N, Hai Y, Liang F, Gao C-J, Liu X-H. Preconditioned hyper-baric oxygenation protects skin flap grafts in rats against ische-mia/reperfusion injury. Mol Med Rep. 2014;9:2124–30. https://doi.org/10.3892/mmr.2014.2064
Hankenson KD, Dishowitz M, Gray C, Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42:556–61. https://doi.org/10.1016/j.injury.2011.03.035
Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovasc Surg. 2005;39:293.
Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60.
Velazquez OC. Angiogenesis and vasculogenesis: Inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45(Suppl. A):A39–A47.
Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14.
Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6.
Hirschi and Goodell. Common origins of blood and blood vessels in adults. Differentiation. 2001;68:186–92.
Barros JW, Barbieri CH, Fernandes CD. Scintigraphic evaluation of tibial shaft fracture healing. Injury. 2000;31:51–54.
Vunjak-Novakovic G, Kaplan DL. Tissue engineering: the next generation. Tissue Eng. 2005;12:3261–3.
Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016;151:e162775. https://doi.org/10.1001/jamasurg.2016.2775
Jeffcoach DR, Sams VG, Lawson CM, Enderson BL, Smith ST, Kline H, et al. Nonsteroidal anti-inflammatory drugs’ impact on nonunion and infection rates in long-bone fractures. J Trauma Acute Care Surg. 2014;76:779–83. https://doi.org/10.1097/TA.0b013e3182aafe0d
Irwin TJ, Orgill D. Closed incision negative pressure wound therapy after resection of large, radiated, soft tissue sarcomas. Cureus. 2020;12:e8055. https://doi.org/10.7759/cureus.8055
Pak CS, Moon SY, Lee YE, Kang HJ. Therapeutic effects against tissue necrosis of remote ischemic preconditioning combined with human adipose-derived stem cells in random-pattern skin flap rat models. J Investig Surg. 2020;34:1304–11. https://doi.org/10.1080/08941939.2020.1795750
Warnke PH, Wiltfang J, Springer I, Acil Y, Bolte H, Kosmahl M, et al. Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials. 2006;27:3163–7.
Heliotis M, Lavery KM, Ripamonti U, Tsiridis E, di Silvio L. Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest. Int J Oral Maxillofac Surg. 2006;35:265–9.
Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker C-H, Walsh A, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96.
Liu F, Porter RM, Wells J, Glatt V, Pilapil C, Evans CH. Evaluation of BMP-2 gene-activated muscle grafts for cranial defect repair. J Orthop Res. 2012;30:1095.
Alfotawi R, Alfayez M, Mahmood A. In situ tissue engineering using an induced muscle graft to reconstruct critical size bone defect. J Biomater Tissue Eng. 2017;7:1114–21.
Al-Fotawei R, Ayoub AF, Heath FN, Naudi KB, Tanner KE, Dalby MJ, et al. Radiological assessment of bio- engineered bone in a muscle flap for reconstruction of a critical-size mandibular defect. PLoS One. 2014;9:e107403.
Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells—Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204.
Bauza-Mayol G, Quintela M, Brozovich A, Hopson M, Shaikh S, Cabrera F, et al. Biomimetic scaffolds modulate the post-traumatic inflammatory response in articular cartilage contributing to enhanced neoformation of cartilaginous tissue in vivo. Adv Healthc Mater. 2022;11:e2101127.
Alfotawi R, Ayoub AF, Tanner KE, Dalby M, Naudi KB, McMahon J. A novel surgical approach for the reconstruction of critical-size mandibular defects using calcium sulphate/hydroxyapatite cement, BMP-7 and Mesenchymal stem cells- Histological assessment. J Biomater Tissue Eng. 2016;6:1–6.
Porzionato A, Sfriso MM, Pontini A, Macchi V, Petrelli L, Pavan PG, et al. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int J Mol Sci. 2015;16:14808–31.
Al-Fotawi R, Muthurangan M, Siyal A, Premnath S, Al-Fayez M, El-Ghannam A, et al. The use of muscle extracellular matrix (MEM) and SCPC bioceramic for bone augmentation. Biomed Mater. 2020;15:025005.
Goumans M-J, Zwijsen A, Dijke PT, Bailly S. Bone morphogenetic proteins in vascular homeostasis and disease. Cold Spring Harb Perspect Biol. 2018;10:a031989. https://doi.org/10.1101/cshperspect.a031989
Zhai W, Lu H, Chen L, Lin X, Huang Y, Dai K, et al. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 2012;8:341.
Deng Y, Jiang C, Li C, Li T, Peng M, Wang J, et al. 3D printed scaffolds of calcium silicate-doped β-TCP synergize with co-cultured endothelial and stromal cells to promote vascularization and bone formation. Sci Rep. 2017;7:1–14.
Joshi A, Choudhury S, Gugulothu SB, Visweswariah SS, Chatterjee K. Strategies to promote vascularization in 3D printed tissue scaffolds: trends and challenges. Biomacromolecules. 2022;23:2730–2751.
Liu H, Du Y, Yang G, Hu X, Wang L, Liu B, et al. Delivering proangiogenic factors from 3D-printed polycaprolactone scaffolds for vascularized bone regeneration. Adv Healthc Mater. 2020;9:2000727.
Duan J, Lei D, Ling C, Wang Y, Cao Z, Zhang M, et al. Three-dimensional-printed polycaprolactone scafolds with interconnected hollow-pipe structures for enhanced bon regenertion. Regen Biomater. 2022;30:1–9.
Yan Y, Chen H, Zhang H, Guo C, Yang K, Chen K, et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials. 2019;190–191:97–110.
Wang C, Lai J, Li K, Zhu S, Lu B, Liu J, et al. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact Mater. 2020;6:137–45.
Lee S, Lee HS, Chung JJ, Kim SH, Park JW, Lee K, et al. Enhanced regeneration of vascularized adipose tissue with dual 3D-printed elastic polymer/DECM hydrogel complex. Int J Mol Sci. 2021;22:2886.
Makhoul N. Development of a microcapillary system for tissue engineering. J Oral Maxillofac Surg. 2008;66:45–46.
Zhang W, Feng C, Yang G, Li G, Ding X, Wang S, et al. 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials. 2017;135:85–95.
Moghadam HG, Urist MR, Sandor GK, Clokie CM. Successful mandibular reconstruction using a BMP bioimplant. J Craniofacial Surg. 2001;12:119–27.
Ferretti C, Ripamonti U. Human segmental mandibular defects treated with naturally derived bone morphogenetic proteins. J Craniofacial Surg. 2002;13:434–44.
Marx RE, Armentano L, Olavarria A, Samaniego J. rhBMP-2/ACS grafts versus autogenous cancellous marrow grafts in large vertical defects of the maxilla: an unsponsored randomized open-label clinical trial. Int J Oral Maxillofac Implants. 2013;28:e243–e251.
Chao M, Donovan T, Sotelo C, Carstens MH. In situ osteogenesis of hemimandible with rhBMP-2 in a 9-year-old boy: osteoinduction via stem cell concentration. J Craniofacial Surg. 2006;17:405–12.
Herford AS, Boyne PJ, Rawson R, Williams RP. Bone morphogenetic protein- induced regeneration of the premaxillary cleft. J Oral Maxillofac Surg. 2007;65:2136–41.
Cicciù M, Herford AS, Stoffella E, Cervino G, Cicciù D. Protein-signaled guided bone regeneration using titanium mesh and Rh-BMP2 in oral surgery: a case report involving left mandibular reconstruction after tumor resection. Open Dent J. 2012;6:51–55.
Ayoub A, Gillgrass T. The clinical application of recombinant human bone morphogenetic protein 7 for reconstruction of alveolar cleft; 10 years follow up. J Oral Maxillofac Surg. 2019;77:571–81. https://doi.org/10.1016/j.joms.2018.08.031
Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008;29:3053–61.
Jin Q, Kim H, Na J, Jin C, Seon J. Anti-infammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci Rep. 2022;12:4754.
Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–43.
Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, et al. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl Med. 2016;5:440–50.
Razban V, Lotfi AS, Soleimani M, Ahmadi H, Massumi M, Khajeh S, et al. HIF-1α overexpression induces angiogenesis in mesenchymal stem cells. BioRes Open Access. 2012;1:174–83. https://doi.org/10.1089/biores.2012.9905
Kinoshita Y, Kobayashi M, Hidaka T, Ikada Y. Reconstruction of mandibular continuity defects in dogs using poly (L-lactide) mesh and autogenic particulate cancellous bone and marrow: Preliminary report. J Oral Maxillofac Surg. 1997;55:718–23.
Lee T-J, Kang S-W, Bhang SH, Kang JM, Kim B-S. Apatite-coated porous poly (lactic-co-glycolic acid) microspheres as an injectable bone substitute. J Biomater Sci Polym Ed. 2010;21:635–45.
Hernandez-Alfaro F, Ruiz-Magaz V, Chatakun P, Guijarro-Martinez R. Mandibular reconstruction with tissue engineering in multiple recurrent ameloblastoma. Int J Periodontics Restor Dent. 2012;32:e82–e86.
Zamiri B, Shahidi S, Eslaminejad MB, Khoshzaban A, Gholami M, Bahramnejad E, et al. Reconstruction of human mandibular continuity defects with allogenic scaffold and autologous marrow mesenchymal stem cells. J Craniofacial Surg. 2013;24:1292–7.
Kim BC, Yoon J-H, Choi B, Lee J. Mandibular reconstructio with autologous human bone marrow stem cells and autogenous bone graft in a patient with plexiform ameloblastoma. J Craniofacial Surg. 2013;24:e409–e411.
Wolff J, Sandor GK, Miettinen A, Tuovinen VJ, Mannerstrom B, Patrikoski M. et al. GMP-level adipose stem cells combined with computer-aided manufacturing to reconstruct mandibular ameloblastoma resection defects: Experience with three cases. Ann Maxillofac Surg. 2013;3:114–25.
Sandor GK, Tuovinen VJ, Wolff J, Patrikoski M, Jokinen J, Nieminen E, et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice- level adipose stem cells for bone regeneration. J Oral Maxillofac Surg. 2013;71:938–50.
Funding
The authors are grateful to the Deanship of Scientific Research, King Saud University for the funding received through the Vice-Deanship of Scientific Research Chairsno RG-1439–062.
Author information
Authors and Affiliations
Contributions
RA wrote the manuscript, data collection and literature review, WF, involved in major revision of the manuscript, editing and proof reading. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Consent for publication
All authors have given consent to publish the study
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AL-Fotawi, R., Fallatah, W. Revascularization and angiogenesis for bone bioengineering in the craniofacial region: a review. J Mater Sci: Mater Med 34, 30 (2023). https://doi.org/10.1007/s10856-023-06730-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-023-06730-6