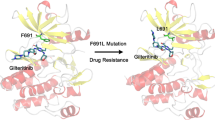
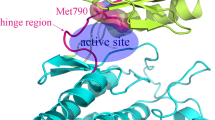
Abstract
FGFR3 kinase mutations are associated with a variety of malignancies, but FGFR3 mutant inhibitors have rarely been studied. Furthermore, the mechanism of pan-FGFR inhibitors resistance caused by kinase domain mutations is still unclear. In this study, we try to explain the mechanism of drug resistance to FGFR3 mutation through global analysis and local analysis based on molecular dynamics simulation, binding free energy analysis, umbrella sampling and community network analysis. The results showed that FGFR3 mutations caused a decrease in the affinity between drugs and FGFR3 kinase, which was consistent with the reported experimental results. Possible mechanisms are that mutations affect drug-protein affinity by altering the environment of residues near the hinge region where the protein binds to the drug, or by affecting the A-loop and interfering with the allosteric communication networks. In conclusion, we systematically elucidated the underlying mechanism of pan-FGFR inhibitor resistance caused by FGFR3 mutation based on molecular dynamics simulation strategy, which provided theoretical guidance for the development of FGFR3 mutant kinase inhibitors.






Similar content being viewed by others
References
Chen L, Yin ZYL et al (2021) Fibroblast growth factor receptor fusions in cancer: opportunities and challenges. J Exp Clin Cancer Res 40:345. https://doi.org/10.1186/s13046-021-02156-6
Krook MA, Reeser JW, Ernst G et al (2021) Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer 124:880–892. https://doi.org/10.1038/s41416-020-01157-0
Savarirayan R, De Bergua JM, Arundel P et al (2022) Infigratinib in children with achondroplasia: the PROPEL and PROPEL 2 studies. Ther Adv Musculoskelet Dis 14:1759720X221084848. https://doi.org/10.1177/1759720X221084848
Babina IS, Turner NC (2017) Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer 17:318–332. https://doi.org/10.1038/nrc.2017.8
Markham A (2019) Erdafitinib: First Global approval. Drugs 79:1017–1021. https://doi.org/10.1007/s40265-019-01142-9
Hoy SM (2020) Pemigatinib: First Approval Drugs 80:923–929
Yu J, Mahipal A, Kim R (2021) Targeted therapy for Advanced or Metastatic Cholangiocarcinoma: Focus on the clinical potential of Infigratinib. OncoTargets Ther 14:5145–5160. https://doi.org/10.2147/OTT.S272208
Subbiah V, Iannotti NO, Gutierrez M et al (2022) FIGHT-101, a first-in-human study of potent and selective FGFR 1–3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann Oncol 33:522–533. https://doi.org/10.1016/j.annonc.2022.02.001
Mahipal A, Tella SH, Kommalapati A, Yu J, Kim R (2020) Prevention and treatment of FGFR inhibitor-associated toxicities. Crit Rev Oncol Hematol 155:103091. https://doi.org/10.1016/j.critrevonc.2020.103091
Mellor HR (2014) Targeted inhibition of the FGF19-FGFR4 pathway in hepatocellular carcinoma; translational safety considerations. Liver Int 34:e1–9. https://doi.org/10.1111/liv.12462
Gattineni J, Bates C, Twombley K et al (2009) FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol 297:F282–291. https://doi.org/10.1152/ajprenal.90742.2008
Haigis KM, Cichowski K, Elledge SJ (2019) Tissue-specificity in cancer: the rule, not the exception. Science 363:1150–1151. https://doi.org/10.1126/science.aaw3472
ICGC/TCGA (2020) Pan-cancer analysis of whole genomes. Nature 578:82–93. https://doi.org/10.1038/s41586-020-1969-6
Pao W, Miller V, Zakowski M et al (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101:13306–13311. https://doi.org/10.1073/pnas.0405220101
Sordella R, Bell DW, Haber DA, Settleman J (2004) Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305:1163–1167. https://doi.org/10.1126/science.1101637
Wang F, Dong X, Yang F, Xing N (2022) Comparative analysis of differentially mutated genes in non-muscle and muscle-invasive bladder Cancer in the Chinese Population by whole exome sequencing. Front Genet 13:831146. https://doi.org/10.3389/fgene.2022.831146
Roubal K, Myint ZW, Kolesar JM (2020) Erdafitinib: a novel therapy for FGFR-mutated urothelial cancer. Am J Health Syst Pharm 77:346–351. https://doi.org/10.1093/ajhp/zxz329
Chandrani P, Prabhash K, Prasad R et al (2017) Drug-sensitive FGFR3 mutations in lung adenocarcinoma. Ann Oncol 28:597–603. https://doi.org/10.1093/annonc/mdw636
Ota N, Yoshimoto Y, Darwis NDM et al (2022) High tumor mutational burden predicts worse prognosis for cervical cancer treated with radiotherapy. Jpn J Radiol 40:534–541. https://doi.org/10.1007/s11604-021-01230-5
Rosty C, Aubriot MH, Cappellen D et al (2005) Clinical and biological characteristics of cervical neoplasias with FGFR3 mutation. Mol Cancer 4:15. https://doi.org/10.1186/1476-4598-4-15
Cohen P, Cross D, Jänne PA (2021) Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discov 20:551–569. https://doi.org/10.1038/s41573-021-00195-4
Rosenzweig SA (2018) Acquired resistance to drugs targeting tyrosine kinases. Adv Cancer Res 138:71–98. https://doi.org/10.1016/bs.acr.2018.02.003
Goyal L, Saha SK, Liu LY et al (2017) Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov 7:252–263. https://doi.org/10.1158/2159-8290.CD-16-1000
Chell V, Balmanno K, Little AS et al (2013) Tumour cell responses to new fibroblast growth factor receptor tyrosine kinase inhibitors and identification of a gatekeeper mutation in FGFR3 as a mechanism of acquired resistance. Oncogene 32:3059–3070. https://doi.org/10.1038/onc.2012.319
Patani H, Bunney TD, Thiyagarajan N et al (2016) Landscape of activating cancer mutations in FGFR kinases and their differential responses to inhibitors in clinical use. Oncotarget 7:24252–24268. https://doi.org/10.18632/oncotarget.8132
Chen L, Marsiglia WM, Chen H, Katigbak J, Mohammadi M (2020) Molecular basis for receptor tyrosine kinase a-loop tyrosine transphosphorylation. Nat Chem Biol 16:1–11. https://doi.org/10.1038/s41589-019-0455-7
A.Waterhouse M, Bertoni S, Bienert et al (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. https://doi.org/10.1093/nar/gky427
Schrodinger LLC (2015) The PyMOL Molecular Graphics System, Version 2.5.
Abraham MJ, Murtola T, Schulz R et al (2015) Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers - sciencedirect. SoftwareX, s1-2 19–25, https://doi.org/10.1016/j.softx.2015.06.001
Huang J, Mackerell AD (2013) Charmm36 all-atom additive protein force field: validation based on comparison to nmr data. J Comput Chem 34:2135–2145. https://doi.org/10.1002/jcc.23354
Tucker JA, Klein T, Breed J, Breeze AL, Norman RA (2014) Structural insights into FGFR kinase isoform selectivity: diverse binding modes of AZD4547 and ponatinib in complex with FGFR1 and FGFR4. Structure 22:1764–1774. https://doi.org/10.1016/j.str.2014.09.019
Bunney TD, Wan S, Thiyagarajan N et al (2015) The effect of mutations on drug sensitivity and kinase activity of fibroblast growth factor receptors: a combined experimental and theoretical study. EBioMedicine 2:194–204. https://doi.org/10.1016/j.ebiom.2015.02.009
Vanommeslaeghe K, Hatcher E, Acharya C, CHARMM General Force Field et al (2010) A Force field for Drug-Like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Field. J Comput Chem 31:671–690. https://doi.org/10.1002/jcc.21367
Yu W, He X, Vanommeslaeghe K, MacKerell AD Jr (2012) Extension of the CHARMM General Force Field to Sulfonyl-Containing Compounds and its utility in Biomolecular Simulations. J Comput Chem 33:2451–2468. https://doi.org/10.1002/jcc.23067
Vanommeslaeghe K, MacKerell AD Jr (2012) Automation of the CHARMM General Force Field (CGenFF) I: bond perception and atom typing. J Chem Inf Model 52:3144–3154. https://doi.org/10.1021/ci300363c
Vanommeslaeghe K, Raman EP, MacKerell AD Jr (2012) Automation of the CHARMM General Force Field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J Chem Inf Model 52:3155–3168. https://doi.org/10.1021/ci3003649
Miller BR 3rd, McGee TD Jr, Swails JM, Homeyer N, Gohlke H, Roitberg AE (2012) MMPBSA.py: an efficient program for end-state Free Energy Calculations. J Chem Theory Comput 8:3314–3321. https://doi.org/10.1021/ct300418h
Li J (2022) gmxtools Zenodo. https://doi.org/10.5281/zenodo.6408973
Lang EJM, Heyes LC, Jameson GB, Parker EJ Calculated pKa variations expose dynamic allosteric communication networks. J Am Chem Soc 138 (2016) 2036–2045, https://doi.org/10.1021/jacs.5b13134
Guo XY, Qi RP, Xu DG, Liu XH, Yang X (2015) Structural and energetic insight into the interactions between the benzolactam inhibitors and tumor marker HSP90α. Comput Biol Chem 58:182–191. https://doi.org/10.1016/j.compbiolchem.2015.07.013
Kästner J (2011) Umbrella sampling. Wiley Interdisciplinary Reviews: Computational Molecular Science 1:932–942
Souaille M, Roux Bt (2001) Extension to the weighted histogram analysis method: combining umbrella sampling with free energy calculations. Comput Phys Commun 135:40–57. https://doi.org/10.1016/s0010-4655(00)00215-0
Kumar S, Rosenberg JM, Bouzida D, Swendsen RH, Kollman PA (1992) The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J Comput Chem 13:1011–1021. https://doi.org/10.1002/jcc.540130812
Chodera JD, Swope WC, Pitera JW, Seok C, Dill KA (2007) Use of the weighted histogram analysis method for the analysis of simulated and parallel tempering simulations. J Chem Theory Comput 3:26–41. https://doi.org/10.1021/ct0502864
Goetz R, Mohammadi M (2013) Exploring mechanisms of FGF signaling through the lens of structural biology. Nat Rev Mol Cell Biol 14:166–180. https://doi.org/10.1038/nrm3528
Chen H, Marsiglia WM, Cho MK et al (2017) Elucidation of a four-site allosteric network in fibroblast growth factor receptor tyrosine kinases. eLife 6:21137. https://doi.org/10.7554/eLife.21137
Lefebvre C, Rubez G, Khartabil H, Boisson JC, Contreras-García J, Hénon E (2017) Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys Chem Chem Phys 19:17928–17936. https://doi.org/10.1039/c7cp02110k
Lu T, Chen Q (2022) Independent gradient model based on Hirshfeld partition: a new method for visual study of interactions in chemical systems. J Comput Chem 43:539–555. https://doi.org/10.1002/jcc.26812
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Zhang J, Adrián FJ, Jahnke W et al (2010) Targeting bcr-abl by combining allosteric with ATP-binding-site inhibitors. Nature 463:501–506. https://doi.org/10.1038/nature08675
Wylie AA, Schoepfer J, Jahnke W et al (2017) The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature 543:733–737. https://doi.org/10.1038/nature21702
Qureshi R, Ghosh A, Yan H (2020) Correlated motions and dynamics in different domains of EGFR with L858R and T790M mutations. IEEE/ACM Trans Comput Biol Bioinform 19:383–394. https://doi.org/10.1109/TCBB.2020.2995569
Girvan M, Newman ME (2002) Community structure in social and biological networks, Proc. Natl. Acad. Sci. U.S.A. 99 7821–7826, https://doi.org/10.1073/pnas.122653799
Gavine PR, Mooney L, Kilgour E et al (2012) AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res 72:20452056. https://doi.org/10.1158/0008-5472.CAN-11-3034
Trudel S, Li ZH, Wei E et al (2005) CHIR–258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood 105:2941–2948. https://doi.org/10.1182/blood-2004-10-3913
Angibaud PR et al (2014) Discovery of JNJ–42756493, a potent fibroblast growth factor receptor (FGFR) inhibitor using a fragment-based approach. Cancer Res 74 Suppl(abstr 4748). https://doi.org/10.1158/1538-7445.AM2014-4748
Adasme MF, Linnemann KL, Bolz SN et al (2021) PLIP 2021: expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res 49:W530–W534. https://doi.org/10.1093/nar/gkab294
Liu X, Shi D, Zhou S, Liu H, Liu H, Yao X (2012) Molecular dynamics simulations and novel drug discovery. Expert Opin Drug Discov 13(1):23–37. https://doi.org/10.1080/17460441.2018.1403419
Campos SR, Machuqueiro M, Baptista AM (2010) Constant-pH molecular dynamics simulations reveal a β-rich form of the human prion protein. J Phys Chem B 114(39):12692–12700. https://doi.org/10.1021/jp104753t
Chen W, van der Kamp MW, Daggett V (2014) Structural and dynamic properties of the human prion protein. Biophys J 106(5):1152–1163. https://doi.org/10.1016/j.bpj.2013.12.053
Santini S, Derreumaux P (2004) Helix H1 of the prion protein is rather stable against environmental perturbations: molecular dynamics of mutation and deletion variants of PrP (90–231). Cell Mol Life Sci 61:951–960. https://doi.org/10.1007/s00018-003-3455-3
Liu H, Yao X (2010) Molecular basis of the interaction for an essential subunit PA – PB1 in influenza virus RNA polymerase: insights from molecular dynamics simulation and free energy calculation. Mol Pharm 7:75–85. https://doi.org/10.1021/mp900131p
Sharon A, Balaraju T, Bal C (2011) A catalytic 3D model development of HIV-integrase and drug resistance understanding by molecular dynamics simulation. Antiviral Res 90:A43–A44. https://doi.org/10.1021/mp900131p
Pan YL, Liu YL, Chen JZ (2018) Computational Simulation Studies on the binding selectivity of 1-(1H-Benzimidazol-5-yl)-5-aminopyrazoles in complexes with FGFR1 and FGFR4. Molecules 23(4):767. https://doi.org/10.3390/molecules23040767
Fu W, Chen L, Wang Z et al (2017) Theoretical studies on FGFR isoform selectivity of FGFR1/FGFR4 inhibitors by molecular dynamics simulations and free energy calculations. Phys Chem Chem Phys 19(5):3649–3659. https://doi.org/10.1039/c6cp07964d
Dehghanian F, Alavi S (2010) Molecular mechanisms of the anti-cancer drug, LY2874455, in overcoming the FGFR4 mutation-based resistance. Sci Rep 11(1):16593. https://doi.org/10.1038/s41598-021-96159-0
Wu C, Chen X, Chen D et al (2019) Insight into ponatinib resistance mechanisms in rhabdomyosarcoma caused by the mutations in FGFR4 tyrosine kinase using molecular modeling strategies. Int J Biol Macromol 135:294–302. https://doi.org/10.1016/j.ijbiomac.2019.05.138
Changeux JP (2013) The concept of allosteric modulation: an overview. Drug Discov Today Technol 10:e223–e228. https://doi.org/10.1016/j.ddtec.2012.07.007
Ni D, Liu Y, Kong R, Yu Z, Lu S, Zhang J (2022) Computational elucidation of allosteric communication in proteins for allosteric drug design. Drug Discov Today 27(8):2226–2234. https://doi.org/10.1016/j.drudis.2022.03.012
Huang Z, Zhao J, Deng W et al (2018) Identification of a cellularly active SIRT6 allosteric activator. Nat Chem Biol 14:1118–1126. https://doi.org/10.1038/s41589-018-0150-0
Acknowledgements
This work was supported by the Natural Science Foundation of Zhejiang Province, China (Grant No. LGF20B020001); National Natural Science Foundation of China (Grant No. 81402839, 81803580); MOE Key Laboratory of Tumor Molecular Biology (Grant No. 50411651-2020-4) and the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021A1515011184).
Author information
Authors and Affiliations
Contributions
B.L. is responsible for research performing, data analyzing and paper writing. J.D. and X.W. are responsible data analyzing. Y.L. is responsible for script writing. J.W., Q.C. and W.L. are responsible for research designing and revised paper writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, B., Ding, J., Liu, Y. et al. Elucidating the potential effects of point mutations on FGFR3 inhibitor resistance via combined molecular dynamics simulation and community network analysis. J Comput Aided Mol Des 37, 325–338 (2023). https://doi.org/10.1007/s10822-023-00510-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-023-00510-8