Abstract
Background
Enteral ibuprofen was first approved as a prescription drug in 1974 for the US market. An intravenous (IV) ibuprofen formulation is approved for use in children older than 6 months of age, but there are limited studies specifically evaluating the pharmacokinetics and safety in children 1–6 months of age.
Aims
The primary purpose of this study was to evaluate the pharmacokinetics of IV ibuprofen in infants younger than 6 months of age. The secondary objective was to evaluate the safety of single and repeated doses of IV ibuprofen in infants younger than 6 months of age.
Methods
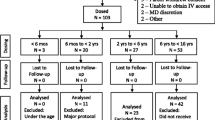
This was an industry-sponsored multi-center study. Institutional Review Board approval and informed parental consent were obtained prior to enrollment. Hospitalized neonates and infants younger than 6 months of age with fever or expected postoperative pain were eligible. Enrolled patients received 10 mg/kg of IV ibuprofen every 6 h, with up to four doses per day. Patients were randomized to two sparse sampling technique pharmacokinetic sample time groups. Group 1 samples were drawn at 0, 30 min, and 2 h, while group 2 samples were drawn at 0 min, 1, and 4 h after administration.
Results
A total of 24 children were enrolled in the study, with 15 male patients and 9 female patients. The median age of the cohort was 4.4 months (range 1.1–5.9 months), and the median weight was 5.9 kg (range 2.3–8.8 kg). The arithmetic mean and standard error for peak plasma ibuprofen concentration was 56.28 ± 2.77 µg/mL. Plasma levels declined rapidly with a mean elimination half-life of 1.30 h. Time to peak ibuprofen effect and concentration were similar when compared with older pediatric patients. Clearance and volume of distribution were also similar to those reported in older pediatric patients. No drug-related adverse events were reported.
Conclusions
The pharmacokinetic and short-term safety profiles of IV ibuprofen in pediatric patients 1–6 months of age are comparable to those in children older than 6 months of age.
Trial Registration
Clinicaltrials.gov Trial Registration number and date: NCT02583399—Registered July 2017.


Similar content being viewed by others
References
Rainsford KD. History and development of ibuprofen. In: Ibuprofen: discovery, development and therapeutics. New York: Wiley Blackwell; 2015. p. 1–21. https://doi.org/10.1002/9781118743614.ch1.
Mencía S, Alonso C, Pallás-Alonso C, López-Herce J. Evaluation and treatment of pain in fetuses, neonates and children. Children. 2022. https://doi.org/10.3390/children9111688.
Doria M, Careddu D, Iorio R, Verrotti A, Chiappini E, Barbero GM, et al. Paracetamol and ibuprofen in the treatment of fever and acute mild–moderate pain in children: Italian experts’ consensus statements. Children. 2021. https://doi.org/10.3390/children8100873.
Pierce CA, Voss B. Efficacy and safety of ibuprofen and acetaminophen in children and adults: a meta-analysis and qualitative review. Ann Pharmacother. 2010;44(3):489–506. https://doi.org/10.1345/aph.1M332.
Tan E, Braithwaite I, Mckinlay CJD, Dalziel SR. Comparison of acetaminophen (paracetamol) with ibuprofen for treatment of fever or pain in children younger than 2 years: a systematic review and meta-analysis. JAMA Netw Open. 2020. https://doi.org/10.1001/jamanetworkopen.2020.22398.
Pavliv L, Voss B, Rock A. Pharmacokinetics, safety, and tolerability of a rapid infusion of i.v. ibuprofen in healthy adults. Am J Health-Syst Pharm. 2011;68(1):47–51. https://doi.org/10.2146/ajhp100120.
Vilenchik R, Berkovitch M, Jossifoff A, Ben-Zvi Z, Kozer E. Oral versus rectal ibuprofen in healthy volunteers. J Popul Ther Clin Pharmacol. 2012;19(2):179–86.
Schwartz JI, Chan CC, Mukhopadhyay S, McBride KJ, Jones TM, Adcock S, et al. Cyclooxygenase-2 inhibition by rofecoxib reverses naturally occurring fever in humans. Clin Pharmacol Ther. 1999;65(6):653–60. https://doi.org/10.1016/S0009-9236(99)90087-5.
Smith HS, Voss B. Pharmacokinetics of intravenous ibuprofen: implications of time of infusion in the treatment of pain and fever. Drugs. 2012;72(3):327–37. https://doi.org/10.2165/11599230-000000000-00000.
Southworth S, Peters J, Rock A, Pavliv L. A multicenter, randomized, double-blind, placebo-controlled trial of intravenous ibuprofen 400 and 800 mg every 6 hours in the management of postoperative pain. Clin Ther. 2009;31(9):1922–35. https://doi.org/10.1016/j.clinthera.2009.08.026.
Litalien C, Jacqz-Aigrain E. Risks and benefits of nonsteroidal anti-inflammatory drugs in children. Pediatr Drugs. 2001;3(11):817–58. https://doi.org/10.2165/00128072-200103110-00004.
Bookstaver B, Miller, Norris, Rudisill. Intravenous ibuprofen: the first injectable product for the treatment of pain and fever. J Pain Res. 2010;3:67–79. https://doi.org/10.2147/jpr.s6993.
Capparelli EV. Pharmacologic, pharmacodynamic, and pharmacokinetic considerations with intravenous ibuprofen lysine. J Pediatr Pharmacol Ther. 2007;12(3):158–70. https://doi.org/10.5863/1551-6776-12.3.158.
Fields E. Summary review for regulatory action. Deputy Division Director Summary Review. NDA 22348 Supplement 005. Caldolor pediatric indication [Internet]. 2015 [cited 2023 May 2]. Available from: https://fda.report/media/95403/22348-Ibuprofen-DD-Clinical-PREA.pdf
Caldolor [package insert]. Nashville: Cumberland Pharmaceuticals Inc.; 2021.
Southworth SR, Sellers JA. Narrative summary of recently published literature on intravenous ibuprofen. Clin Ther. 2020;42:1210–21. https://doi.org/10.1016/j.clinthera.2020.05.004.
Khalil SN, Hahn BJ, Chumpitazi CE, Rock AD, Kaelin BA, Macias CG. A multicenter, randomized, open-label, active-comparator trial to determine the efficacy, safety, and pharmacokinetics of intravenous ibuprofen for treatment of fever in hospitalized pediatric patients. BMC Pediatr. 2017;17(1):42. https://doi.org/10.1186/s12887-017-0795-y.
Lovecchio F, Premkumar A, Stepan JG, Albert TJ. fighting back: institutional strategies to combat the opioid epidemic: a systematic review. HSS J. 2019;15(1):66–71. https://doi.org/10.1007/s11420-018-09662-y.
Cui X, Zhang J, Gao Z, Sun L, Zhang F. A randomized, double-blinded, placebo-controlled, single dose analgesic study of preoperative intravenous ibuprofen for tonsillectomy in children. Front Pediatr. 2022. https://doi.org/10.3389/fped.2022.956660.
Patel NK, Shah SJ, Lee NK, Gao Q, Carullo VP, Yang CJ. Intraoperative intravenous ibuprofen use is not associated with increased post-tonsillectomy bleeding. Int J Pediatr Otorhinolaryngol. 2020. https://doi.org/10.1016/j.ijporl.2020.109965.
Abdelbaser I, Abo-Zeid M, Hayes S, Taman HI. The analgesic effects of the addition of intravenous ibuprofen to a multimodal analgesia regimen for pain management after pediatric cardiac surgery: a randomized controlled study. J Cardiothorac Vasc Anesth. 2022. https://doi.org/10.1053/j.jvca.2022.11.016.
Gokmen T, Erdeve O, Altug N, Oguz SS, Uras N, Dilmen U. Efficacy and safety of oral versus intravenous ibuprofen in very low birth weight preterm infants with patent ductus arteriosus. J Pediatr. 2011;158(4):549-554.e1. https://doi.org/10.1016/j.jpeds.2010.10.008.
Aranda JV, Thomas R. Systematic review: intravenous ibuprofen in preterm newborns. Semin Perinatol. 2006;30:114–20. https://doi.org/10.1053/j.semperi.2006.04.003.
Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD003481.pub8.
US Department of Health and Human Services. Guidance for industry bioanalytical method validation. [Internet]. 2018 [cited 2023 Jan 30]. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf.
Kozlowski LJ, Kost-Byerly S, Colantuoni E, Thompson CB, Vasquenza KJ, Rothman SK, et al. Pain prevalence, intensity, assessment and management in a hospitalized pediatric population. Pain Manag Nurs. 2014;15(1):22–35. https://doi.org/10.1016/j.pmn.2012.04.003.
Poddighe D, Brambilla I, Licari A, Marseglia GL. Ibuprofen for pain control in children new value for an old molecule [Internet]. 2018. Available from: http://www.pec-online.com.
Walsh P, Rothenberg SJ, Bang H. Safety of ibuprofen in infants younger than six months: a retrospective cohort study. PLoS ONE. 2018;13(6): e0199493. https://doi.org/10.1371/journal.pone.0199493.
Ziesenitz VC, Welzel T, van Dyk M, Saur P, Gorenflo M, van den Anker JN. Efficacy and safety of NSAIDs in infants: a comprehensive review of the literature of the past 20 years. Pediatr Drugs. 2022;24:603–55. https://doi.org/10.1007/s40272-022-00514-1.
Kauffman RE, Nelson MV. Effect of age on ibuprofen pharmacokinetics and antipyretic response. J Pediatr. 1992;121(6):969–73. https://doi.org/10.1016/s0022-3476(05)80354-3.
Morris PE, Promes JT, Guntupalli KK, Wright PE, Arons MM. A multi-center, randomized, double-blind, parallel, placebo-controlled trial to evaluate the efficacy, safety, and pharmacokinetics of intravenous ibuprofen for the treatment of fever in critically ill and non-critically ill adults. Crit Care. 2010. https://doi.org/10.1186/cc9089.
Aranda J, Varvarigou A, Beharry K, Bansal R, Bardin C, Modanlou H, et al. Pharmacokinetics and protein binding of intravenous ibuprofen in the premature newborn infant. Acta Paediatr. 1997;86(3):289–93. https://doi.org/10.1111/j.1651-2227.1997.tb08892.x.
Van Overmeire B, Touw D, Schepens PJC, Kearns GL, Van Den Anker JN. Ibuprofen pharmacokinetics in preterm infants with patent ductus arteriosus. Clin Pharmacol Ther. 2001;70(4):336–43.
Nahata MC, Durrell DE, Powell DA, Gupta N. Pharmacokinetics of ibuprofen in febrile children. Eur J Clin Pharmacol. 1991. https://doi.org/10.1007/BF00265858.
Edison PE, Chen S, Yeo CL, Allen JC, Poon WB, Baral VR, et al. Pharmacokinetics of oral versus intravenous ibuprofen for closure of patent ductus arteriosus: a pilot randomised controlled study. J Paediatr Child Health. 2022;58(3):397–403. https://doi.org/10.1111/jpc.15720.
Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2):250–67. https://doi.org/10.1016/j.pharmthera.2008.02.005.
Brion LP, Fleischman AR, McCarton C, Schwartz GJ. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: Noninvasive assessment of body composition and growth. J Pediatr. 1986;109(4):698–707. https://doi.org/10.1016/s0022-3476(86)80245-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This clinical trial was sponsored by Cumberland Pharmaceuticals Inc.
Conflict of interest
CDG, JWB, MBT, NVP, and JZ were investigators on this clinical trial. BK and BHYG are employees of Cumberland Pharmaceuticals Inc.
Ethics approval
All studies were conducted following approval by site Institutional Review Boards and performed in accordance with the Declaration of Helsinki.
Consent
Prior to any study-related procedures, written informed consent was obtained from the parent or legal guardian of the patient.
Author contributions
CDG, JWB, MBT, NVP, and JZ were investigators in the clinical study. BK developed study design and BHYG prepared the data. All authors contributed to drafting the manuscript, providing critical revisions, and approving the final manuscript.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Glover, C.D., Berkenbosch, J.W., Taylor, M.B. et al. A Multi-Center Evaluation of the Pharmacokinetics and Safety of Intravenous Ibuprofen in Infants 1–6 Months of Age. Pediatr Drugs 25, 585–593 (2023). https://doi.org/10.1007/s40272-023-00576-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00576-9