Abstract—
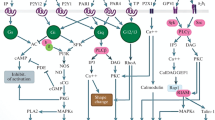
One of the key receptors on the surface of platelets, non-nuclear cells responsible for preventing blood loss when blood vessels are damaged, is the receptor for the extracellular matrix protein collagen, glycoprotein VI (GPVI). GPVI triggers tyrosine kinase signaling in platelets, simultaneously initiating calcium signaling via phospholipase Cγ2 (PLCγ2) and phosphoinositide signaling via phosphoinositide-3-kinase (PI3K). Previously, our group demonstrated that among healthy donors there is more than a twofold variability in calcium response to activation through the GPVI receptor. Here, a computer model of platelet activation through the GPVI receptor is proposed to explain this phenomenon. This model is a system of ordinary differential equations integrated with the LSODA method. The model equations were derived from our previously published model of platelet activation via the CLEC-2 receptor. Using the developed model, a monotonic dependence of the degree of platelet activation on the number of GPVI receptors was predicted. An analysis of the sensitivity of the model to its parameters showed that the platelet response to activation through GPVI is determined by the number of GPVI receptors, as well as the catalytic parameters of tyrosine kinases, while a twofold change in the number of receptors is sufficient to explain the observed phenomenon. Thus, it was theoretically predicted that the variability of calcium responses of platelets to their stimulation through the GPVI receptor could be determined by the variability in the number of GPVI receptors on the platelet surface of healthy donors.



Similar content being viewed by others
REFERENCES
Panteleev M.A., Sveshnikova A.N. 2014. Platelets and hemostasis. Oncohematologia (Rus.). 9 (2), 65–73.
Sveshnikova A., Stepanyan M., Panteleev M., Sveshnikova A., Stepanyan M., Panteleev M. 2021. Platelet functional responses and signalling: The molecular relationship. Part 1: Responses. Systems Biology and Physiology Reports. 1 (1), 20.
Bergmeier W., Stefanini L. 2009. Novel molecules in calcium signaling in platelets. J. Thromb. Haemostasis. 7, 187–190.
Martyanov A., Panteleev M. 2021. Platelet functional responses and signalling: The molecular relationship. Part 2: Receptors. SBPR. 1 (3), 13–30.
Gear A.R. 1994. Platelet adhesion, shape change, and aggregation: Rapid initiation and signal transduction events. Can. J. Physiol. Pharmacol. 72 (3), 285–294.
Kaneva V.N., Martyanov A.A., Morozova D.S., Panteleev M.A., Sveshnikova A.N. 2019. Platelet Integrin αIIbβ3: Mechanisms of activation and clustering; Involvement into the formation of the thrombus heterogeneous structure. Biochem. (Moscow) Suppl. Series A, Membr. Cell Biol. 13 (2), 97–110.
Podoplelova N.A., Sveshnikova A.N., Kotova Y.N., Eckly A., Receveur N., Nechipurenko D.Yu., Obydennyi S.I., Kireev I.I., Gachet C., Ataullakhanov F.I., Mangin P.H., Panteleev M.A. 2016. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood. 128 (13), 1745–1755.
Bryckaert M., Rosa J.-P., Denis C.V., Lenting P.J. 2015. Of von Willebrand factor and platelets. Cell. Mol. Life Sciences, CMLS. 72 (2), 307–326.
Poulter N.S., Pollitt A.Y., Owen D.M., Gardiner E.E., Andrews R.K., Shimizu H., Ishikawa D., Bihan D., Farndale R.W., Moroi M., Watson S.P., Jung S.M. 2017. Clustering of glycoprotein VI (GPVI) dimers upon adhesion to collagen as a mechanism to regulate GPVI signaling in platelets. J. Thromb. Haemost. 15 (3), 549–564.
Stepanyan M.G., Filkova A.A., Dasgupta A.G., Martyanov A.A., Sveshnikova A.N. 2021. Platelet activation through GPVI receptor: Variability of the response. Biochem. (Moscow) Suppl. Series A, Membr. Cell Biol. 15 (1), 73–81.
Johnson E.N., Brass L.F., Funk C.D. 1998. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc. Natl. Acad. Sci. USA. 95 (6), 3100–3105.
Pollitt A.Y., Poulter N.S., Gitz E., Navarro-Nuñez L., Wang Y.-J., Hughes C.E., Thomas S.G., Nieswandt B., Douglas M.R., Owen D.M., Jackson D.G., Dustin M.L., Watson S.P. 2014. Syk and Src family kinases regulate C-type lectin receptor 2 (CLEC-2)-mediated clustering of podoplanin and platelet adhesion to lymphatic endothelial cells. J. Biol. Chem. 289 (52), 35695–35710.
Garzon Dasgupta A.K., Martyanov A.A., Filkova A.A., Panteleev M.A., Sveshnikova A.N. 2020. Development of a simple kinetic mathematical model of aggregation of particles or clustering of receptors. Life. 10 (6), 97.
Watson S.P., Herbert J.M.J., Pollitt A.Y. 2010. GPVI and CLEC-2 in hemostasis and vascular integrity. J. Thromb. Haemost. 8 (7), 1456–1467.
Rayes J., Watson S.P., Nieswandt B. 2019. Functional significance of the platelet immune receptors GPVI and CLEC-2. J. Clin. Invest. 129 (1), 12–23.
Balabin F.A., Sveshnikova A.N. 2016. Computational biology analysis of platelet signaling reveals roles of feedbacks through phospholipase C and inositol 1,4,5-trisphosphate 3-kinase in controlling amplitude and duration of calcium oscillations. Math. Biosci. 276, 67–74.
Sveshnikova A.N., Balatskiy A.V., Demianova A.S., Shepelyuk T.O., Shakhidzhanov S.S., Balatskaya M.N., Pichugin A.V., Ataullakhanov F.I., Panteleev M.A. 2016. Systems biology insights into the meaning of the platelet’s dual-receptor thrombin signaling. J. Thromb. Haemost. 14 (10), 2045–2057.
Jackson S.P., Schoenwaelder S.M. 2010. Procoagulant platelets: Are they necrotic? Blood. 116 (12), 2011–2018.
Podoplelova N.A., Nechipurenko D.Y., Ignatova A.A., Sveshnikova A.N., Panteleev M.A. 2021. Procoagulant platelets: Mechanisms of generation and action. Hämostaseologie. 41 (02), 146–153.
Martyanov A.A., Balabin F.A., Dunster J.L., Panteleev M.A., Gibbins J.M., Sveshnikova A.N. 2020. Control of platelet CLEC-2-mediated activation by receptor clustering and tyrosine kinase signaling. Biophys. J. 118 (11), 2641–2655.
Moroi M., Jung S.M. 2004. Platelet glycoprotein VI: Its structure and function. Thromb. Res. 114 (4), 221–233.
Furihata K., Clemetson K.J., Deguchi H., Kunicki T.J. 2001. Variation in human platelet glycoprotein VI content modulates glycoprotein VI-specific prothrombinase activity. Arterioscler. Thromb. Vasc. Biol. 21 (11), 1857–1863.
Best D., Senis Y.A., Jarvis G.E., Eagleton H.J., Roberts D.J., Saito T., Jung S.M., Moroi M., Harrison P., Green F.R., Watson S.P. 2003. GPVI levels in platelets: Relationship to platelet function at high shear. Blood. 102 (8), 2811–2818.
Hoops S., Sahle S., Gauges R., Lee C., Pahle J., Simus N., Singhal M., Xu L., Mendes P., Kummer U. 2006. COPASI – a COmplex PAthway SImulator. Bioinformatics. 22 (24), 3067–3074.
Petzold L., Hindmarsh A. 1997. LSODA (Livermore solver of ordinary differential equations). Computing and Mathematics Research Division, Lawrence Livermore National Laboratory, Livermore, CA, 24.
Burkhart J.M., Vaudel M., Gambaryan S., Radau S., Walter U., Martens L., Geiger J., Sickmann A., Zahedi R.P. 2012. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 120 (15), e73–e82.
Back T. 1996. Evolutionary algorithms in theory and practice: Evolution strategies, evolutionary programming, genetic algorithms. Oxford: Oxford University Press.
Saltelli A., Ratto M., Tarantola S., Campolongo F. 2005. Sensitivity analysis for chemical models. Chem. Rev. 105 (7), 2811–2828.
Martyanov A.A., Balabin F.A., Mayorov A.S., Shamova E.V., Panteleev M.A., Sveshnikova A.N. 2018. Computer simulation of intracellular signalling during activation of blood platelets by fucoidan. Biol. Membrany (Rus.). 35 (5), 364–375.
Dunster J.L., Mazet F., Fry M.J., Gibbins J.M., Tindall M.J. 2015. Regulation of early steps of GPVI signal transduction by phosphatases: A systems biology approach. PLoS Comput. Biol. 11 (11), e1004589.
Rayes J., Watson S.P., Nieswandt B. 2019. Functional significance of the platelet immune receptors GPVI and CLEC-2. J. Clin. Invest. 129 (1), 12–23.
Senis Y.A., Mazharian A., Mori J. 2014. Src family kinases: At the forefront of platelet activation. Blood. 124 (13), 2013–2024.
Séverin S., Pollitt A.Y., Navarro-Nuñez L., Nash C.A., Mourão-Sá D., Eble J.A., Senis Y.A., Watson S.P. 2011. Syk-dependent phosphorylation of CLEC-2. J. Biol. Chem. 286 (6), 4107–4116.
Burkhart J.M., Vaudel M., Gambaryan S., Radau S., Walter U., Martens L., Geiger J., Sickmann A., Zahedi R.P. 2012. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 120 (15), e73–e82.
Quinter P.G., Quinton T.M., Dangelmaier C.A., Kunapuli S.P., Daniel J.L. 2005. Role of lipid rafts in GPVI agonist-induced platelet signaling. Blood. 106 (11), 3576.
Miura Y., Takahashi T., Jung S.M., Moroi M. 2002. Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. J. Biol. Chem. 277 (48), 46197–46204.
Kemble D.J., Wang Y.H., Sun G. 2006. Bacterial expression and characterization of catalytic loop mutants of Src protein tyrosine kinase. Biochemistry. 45 (49), 14749–14754.
Bradshaw J.M. 2010. The Src, Syk, and Tec family kinases: Distinct types of molecular switches. Cell. Signal. 22 (8), 1175–1184.
Ren L., Chen X., Luechapanichkul R., Selner N.G., Meyer T.M., Wavreille A.-S., Chan R., Iorio C., Zhou X., Neel B.G., Pei D. 2011. Substrate specificity of protein tyrosine phosphatases 1B, RPTPα, SHP-1, and SHP-2. Biochemistry. 50 (12), 2339–2356.
Lin X., Lee S., Sun G. 2003. Functions of the activation loop in Csk protein-tyrosine kinase. J. Biol. Chem. 278 (26), 24072–24077.
Park M.J., Sheng R., Silkov A., Jung D.J., Wang Z.G., Xin Y., Kim H., Thiagarajan-Rosenkranz P., Song S., Yoon Y., Nam W., Kim I., Kim E., Lee D.G., Chen Y., Singaram I., Wang L., Jang M.H., Hwang C.S., Honig B., Ryu S., Lorieau J., Kim Y.M., Cho W. 2016. SH2 domains serve as lipid-binding modules for pTyr-signaling proteins. Mol. Cell. 62 (1), 7–20.
Tsang E., Giannetti A.M., Shaw D., Dinh M., Tse J.K.Y., Gandhi S., Ho A., Wang S., Papp E., Bradshaw J.M. 2008. Molecular mechanism of the Syk activation switch. J. Biol. Chem. 283 (47), 32650–32659.
Hughes C.E., Sinha U., Pandey A., Eble J.A., O’Callaghan C.A., Watson S.P. 2013. Critical role for an acidic amino acid region in platelet signaling by the HemITAM (hemi-immunoreceptor tyrosine-based activation motif) containing receptor CLEC-2 (C-type lectin receptor-2). J. Biol. Chem. 288 (7), 5127–5135.
Sveshnikova A.N., Balatskiy A.V., Demianova A.S., Shepelyuk T.O., Shakhidzhanov S.S., Balatskaya M.N., Pichugin A.V., Ataullakhanov F.I., Panteleev M.A. 2016. Systems biology insights into the meaning of the platelet’s dual-receptor thrombin signaling. J. Thromb. Haemost. 14 (10), 2045–2057.
Ahmed M.U., Kaneva V., Loyau S., Nechipurenko D., Receveur N., Le Bris M., Janus-Bell E., Didelot M., Rauch A., Susen S., Chakfé N., Lanza F., Gardiner E.E., Andrews R.K., Panteleev M., Gachet C., Jandrot-Perrus M., Mangin P.H. 2020. Pharmacological blockade of glycoprotein VI promotes thrombus disaggregation in the absence of thrombin. Arterioscler. Thromb. Vasc. Biol. 40 (9), 2127–2142.
Montague S.J., Delierneux C., Lecut C., Layios N., Dinsdale R.J., Lee C.S.-M., Poulter N.S., Andrews R.K., Hampson P., Wearn C.M., Maes N., Bishop J., Bamford A., Gardiner C., Lee W.M., Iqbal T., Moiemen N., Watson S.P., Oury C., Harrison P., Gardiner E.E. 2018. Soluble GPVI is elevated in injured patients: Shedding is mediated by fibrin activation of GPVI. Blood Adv. 2 (3), 240–251.
Montague S.J., Andrews R.K., Gardiner E.E. 2018. Mechanisms of receptor shedding in platelets. Blood. 132 (24), 2535–2545.
Al-Tamimi M., Tan C.W., Qiao J., Pennings G.J., Javadzadegan A., Yong A.S.C., Arthur J.F., Davis A.K., Jing J., Mu F.-T., Hamilton J.R., Jackson S.P., Ludwig A., Berndt M.C., Ward C.M., Kritharides L., Andrews R.K., Gardiner E.E. 2012. Pathologic shear triggers shedding of vascular receptors: A novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood. 119 (18), 4311–4320.
Yakusheva A.A., Butov K.R., Bykov G.A., Závodszky G., Eckly A., Ataullakhanov F.I., Gachet C., Panteleev M.A., Mangin P.H. 2022. Traumatic vessel injuries initiating hemostasis generate high shear conditions. Blood Adv. 6 (16), 4834–4846.
Funding
This work was supported by the Russian Foundation for Basic Research and London Royal Community (project no. 21-51-10005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Martyanov
Abbreviations: CRP, collagen related peptide; DTS, dense tubular system; ER, endoplasmic reticulum; GPVI, glycoprotein VI; IP3, inositol-1,4,5-triphosphate; IP3R, IP3 receptor; LAT, linker adapter T-cell protein; PLCγ2, phospholipase Cγ2; PMCA, plasma membrane calcium ATPase; PRP, platelet rich plasma; SERCA, sarcoplasmic/endoplasmic reticulum calcium ATPase; VWF, von Willebrand factor.
Rights and permissions
About this article
Cite this article
Martyanov, A.A., Stepanyan, M.G. & Sveshnikova, A.N. Theoretical Explanation for the Variability in Platelet Activation through the GPVI Receptor. Biochem. Moscow Suppl. Ser. A 17, 83–91 (2023). https://doi.org/10.1134/S1990747823020046
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747823020046