Abstract
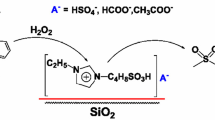
A comparative analysis is performed for the properties of two Fenton-type catalysts in the oxidation of sulfur-containing compounds with hydrogen peroxide and the desulfurization of crude oil. The catalysts are based on Cu(I) and Fe(III), and Mo(VI) and W(VI) polyoxometalates. Heterogeneous samples are imidazolium salts chemically immobilized on surfaces of silochrome and contain the iron and copper chloride complexes or phosphomolybdic and phosphotungstic acid anions. Thiophene (T), dibenzothiophene (DBT), methylphenyl sulfide (MPS), and a diesel fraction with an initial amount of sulfur of 1080 ppm are used as model substrates. It is found that the order of reactivity of thiophene substrates depends on the nature of metal-containing anions: thiophene > DBT on the Cu and Fe catalysts and DBT > thiophene on the polyoxometalate catalysts. This effect is explained using literature data. The catalyst based on phosphotungstic acid allows desulfurization of the diesel fraction of oil to amounts of sulfur of < 10 ppm, which meets today’s environmental standards.




Similar content being viewed by others
REFERENCES
Tanimu, A. and Alhooshani, K., Energy Fuels, 2019, vol. 33, no. 4, pp. 2810–2838. https://doi.org/10.1021/acs.energyfuels.9b00354
Rajendran, A., Cui, T., Fan, H., Yang, Z., Feng, J., and Li, W., J. Mater. Chem. A, 2020, vol. 8, no. 5, pp. 2246–2285. https://doi.org/10.1039/C9TA12555H
Eseva, E.A., Akopyan, A.V., Anisimov, A.V., and Maksimov, A.L., Pet. Chem., 2020, vol. 60, no. 9, pp. 979–990. https://doi.org/10.1134/S0965544120090091
Abdullah, S.B., Aziz, H.A., and Man, Z., in Recent Advances in Ionic Liquids, Rahman, M.M., Ed., London: IntechOpen 2018, pp. 107–120. https://doi.org/10.5772/intechopen.79281.
Ibrahim, M.H., Hayyan, M., Hashim, M.A., and Hayyan, A., Renewable Sustainable Energy Rev., 2017, vol. 76, pp. 1534–1549. https://doi.org/10.1016/j.rser.2016.11.194
Romanovskii, B.V. and Tarkhanova, I.G., Russ. Chem. Rev., 2017, vol. 86, no. 5, pp. 444–458. https://doi.org/10.1070/RCR4666
Bryzhin, A.A., Rudnev, V.S., Lukiyanchuk, I.V., Vasil’eva, M.S., and Tarkhanova, I.G., Kinet. Catal., 2020, vol. 61, no. 2, pp. 283–290. https://doi.org/10.1134/S0023158420020020
Xun, S., Zhu, W., Chang, Y., Li, H., Zhang, M., Jiang, W., Zheng, D., Qin, Y., and Li, H., Chem. Eng. J., 2016, vol. 288, pp. 608–617. https://doi.org/10.1016/j.cej.2015.12.005
Li, X., Zhang, J., Zhou, F., Wang, Y., Yuan, X., and Wang, H., Mol. Catal., 2018, vol. 452, pp. 93–99. https://doi.org/10.1016/j.mcat.2017.09.038
Abdullah, W.N.W., Bakar, W.A.W.A., Ali, R., Mokhtar, W.N.A.W., and Omar, M.F., J. Cleaner Prod., 2017, vol. 162, pp. 1455–1464. https://doi.org/10.1016/j.jclepro.2017.06.084
Hao, Y., Hao, Y., Ren, J., Wu, B., Wang, X., Zhao, D., and Li, F., New J. Chem., 2019, vol. 43, no. 20, pp. 7725–7732. https://doi.org/10.1039/C9NJ00691E
Ivanin, I.A., Ali-Zade, A.G., Golubeva, E.N., Zubanova, E.M., Zelikman, V.M., Buryak, A.K., and Tarkhanova, I.G., Mol. Catal., 2020, vol. 484, article no. 110727. https://doi.org/10.1016/j.mcat.2019.110727
Ali-Zade, A.G., Buryak, A.K., Zelikman, V.M., Oskolok, K.V., and Tarkhanova, I.G., New J. Chem., 2020, vol. 44, no. 16, pp. 6402–6410. https://doi.org/10.1039/C9NJ05403K
Baltrusaitis, J., Mendoza-Sanchez, B., Fernandez, V., Veenstra, R., Dukstiene, N., Roberts, A., and Fairley, N., Appl. Surf. Sci., 2015, vol. 326, pp. 151–161. https://doi.org/10.1016/j.apsusc.2014.11.077
Jalil, P.A., Faiz, M., Tabet, N., Hamdan, N.M., and Hussain, Z., J. Catal., 2003, vol. 217, no. 2, pp. 292–297. https://doi.org/10.1016/S0021-9517(03)00066-6
Zhang, B., Jiang, Z., Li, J., Zhang, Y., Lin, F., Liu, Y., and Li, C., J. Catal., 2012, vol. 287, pp. 5–12. https://doi.org/10.1016/j.jcat.2011.11.003
Craven, M., Xiao, D., Kunstmann-Olsen, C., Kozhevnikova, E.F., Blanc, F., Steiner, A., and Kozhevnikov, I.V., Appl. Catal., B, 2018, vol. 231, pp. 82–91. https://doi.org/10.1016/j.apcatb.2018.03.005
Song, C., Catal. Today, 2003, vol. 86, nos. 1–4, pp. 211–263. https://doi.org/10.1016/S0920-5861(03)00412-7
Ghubayra, R., Nuttall, C., Hodgkiss, S., Craven, M., Kozhevnikova, E.F., and Kozhevnikov, I.V., Appl. Catal., B, 2019, vol. 253, pp. 309–316. https://doi.org/10.1016/j.apcatb.2019.04.063
Li, J., Yang, Z., Li, S., Jin, Q., and Zhao, J., J. Ind. Eng. Chem., 2020, vol. 82, pp. 1–16. https://doi.org/10.1016/j.jiec.2019.10.020
Luna, M.L., Alvarez-Amparán, M.A., and Cedeño-Caero, L., J. Taiwan Inst. Chem. Eng., vol. 95, pp. 175–184. https://doi.org/10.1016/j.jtice.2018.06.010
Feng, Y., Lee, P.-H., Wu, D., Zhou, Z., Li, H., and Shih, K., J. Hazard. Mater., 2017, vol. 331, pp. 81–87. https://doi.org/10.1016/j.jhazmat.2017.02.029
Hwang, S., Huling, S.G., and Ko, S., Chemosphere, 2010, vol. 78, no. 5, pp. 563–568. https://doi.org/10.1016/j.chemosphere.2009.11.005
ACKNOWLEDGMENTS
This work was performed on an equipment purchased as part of the Developmental Program of Moscow State University.
Funding
This work was performed as part of state task no. АААА-А21-121011590090-7 for the Moscow State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state they have no conflicts of interest.
Additional information
Translated by A. Tulyabaev
Rights and permissions
About this article
Cite this article
Tarkhanova, I.G., Ali-Zade, A.G., Buryak, A.K. et al. Effect of Metal-Containing Anions on the Catalytic Properties of Imidazolium Derivatives Immobilized on Silochrome in Oxidative Desulfurization. Catal. Ind. 15, 125–131 (2023). https://doi.org/10.1134/S2070050423020101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050423020101