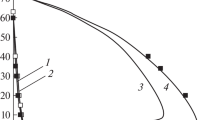
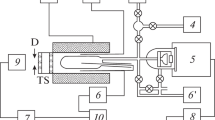
Abstract
It has been established that, contrary to previous ideas, chemical processes in flame propagation, explosion, and detonation of gases are chain reactions proceeding according to previously unknown laws of nonisothermal chain processes. The characteristic reaction times in deflagration and detonation in the combustion zone are less than a ten-thousandth and millionth of a second, respectively. The mechanisms and laws that determine these high rates and accelerations of reactions and their extremely strong temperature dependence were revealed. The critical role of atoms and radicals, formed in concentrations reaching tens of percent of the concentrations of the initial reagents, was shown using kinetic and spectroscopic methods. Efficient chemical methods for controlling all combustion modes were developed.









Similar content being viewed by others
REFERENCES
Semenov, N.N., Usp. Fiz. Nauk, 1940, vol. 23, no. 1, p. 251.
Semenov, N.N., Razvitie teorii tsepnykh reaktsii i teplovogo vosplameneniya (Development of the Theory of Chain Reactions and Thermal Ignition), Moscow: Izd. Znanie, 1969.
Semenov, N.N., Izbrannye trudy (Selected Works), Moscow: Nauka, 2005, vol. 3.
Lewis, B. and Von Elbe, G., Combustion, Explosions, and Flame in Gases, New York: Acad. Press, 1987.
Baulch, D.L., Bowman, C.T., Cobos, C.J., Cox, R.A., Just, T., Kerr, J.A., Pilling, M.J., Stoker, D., Troe, J., Tsang, W., Walker, R.W., and Warnatz, J., J. Phys. Chem. Ref. Data, 2005, vol. 34, no. 3, p. 757.
Srinivasan, N.K., Michael, J.V., Harding, L.B., and Klipperstain, S.J., Combust. Flame, 2007, vol. 149, nos. 1–2, p. 104.
Zel’dovich, Ya.B., Barenblat, G.I., Librovich, V.B., and Makhviladze, G.M., Matematicheskaya teoriya goreniya (Mathematical Theory of Combustion), Moscow: Nauka, 1980.
Williams, F.A., Combustion Theory, Westview Press, 1985.
Combustion, Khimicheskaya entsiklopediya (Chemical Encyclopedia), Moscow: Sov. Entsiklopediya, 1988, vol. 1, p. 595.
Combustion, Fizicheskaya entsiklopediya (Physical Encyclopedia), Moscow: Sov. Entsiklopediya, 1988, p. 515.
Combustion, Bol’shoi entsiklopedicheskii slovar’ “Fizika” (Large Encyclopedic Dictionary), Moscow: Izd. Bol’shaya Rossiiskaya entsiklopediya, 1998, p. 134.
Merzhanov, A.G., Nonequilibrium theory of flame propagation, Prog. Astron. Aeronaut., 1997, vol. 173, p. 37.
Frank-Kamenetskii, D.A., Osnovy makrokinetiki, diffuziya, teploperedacha v khimicheskoi kinetike (Fundamentals of Macrokinetics, Diffusion, Heat Transfer in Chemical Kinetics), Dolgoprudnyi: Intellekt, 2008.
Kukin, P.P., Yushin, V.V., and Emel’yanov, S.G., Teoriya goreniya i vzryva (Theory of Combustion and Explosion), Moscow: Yurait, 2012.
Palesskii, F.S., Fursenko, R.V., and Minaev, S.S., Fiz. Goreniya Vzryva, 2014, vol. 50, no. 6, p. 3.
Frolov, S.M., Shamshin, I.V., Dubrovskii, F.V., and Medvedev, S.N., in Proc. Int. Conf. on Transient Combustion and Detonation, Moscow: Torus press, 2014, p. 204.
Kogan, I. and Sivashinsky, G., Modeling of deflagration-to-detonation transition with ignition temperature kinetics, in Proc. Int. Conf. on Transient Combustion and Detonation Phenomena, Moscow, 2014, p. 163.
Lewis, B. and Von Elbe, G., Combustion, Flames and Explosions of Gases, New York: Acad. Press, 1951.
Dubrovskii, A.V., Ivanov, V.S., Zangiev, A.E., and Frolov, S.M., Khim. Fiz., 2016, vol. 35, no. 6, p. 49.
Warnatz, J., Maas, U., and Dibble, R.W., Combustion: Physical and Chemical Fundamentals, Modeling and Simulation, Experiments, Pollutant Formation, Berlin: Springer, 2001.
Babkin, V.S. and Senachin, P.K., Protsessy goreniya gazov v ogranichennykh ob’’emakh (Gas Combustion Processes in Confined Volumes), Barnaul: Izd. Alt. Gos. Tekh. Univ., 2017.
Chung K. Law, Combustion Physics, Cambridge, NY: Cambridge Univ. Press, 2006.
Azatyan, V.V., Usp. Khim., 1999, vol. 62, no. 12, p. 1122.
Azatyan, V.V., Vagner, G.G., and Vedeshkin, G.K., Zh. Fiz. Khim., 2004, vol. 78, no. 6, p. 1036.
Azatyan, V.V., Pavlov, V.A., and Shatalov, O.P., Kinet. Katal., 2005, vol. 46, no. 6, p. 835.
Azatyan, V.V., Zh. Fiz. Khim., 2011, vol. 85, no. 8, p. 1405.
Azatyan, V.V., Bolod’yan, I.A., Shebeko, Yu.N., and Navtsen’ya, V.Yu., Kinet. Katal., 2003, vol. 27, no. 3, p. 449.
Petrova, L.D., Azatyan, V.V., Baratov, A.N., Vogman, L.P., and Makeev, V.N., in Sb. Gorenie i vzryv (Combustion and Explosion), Moscow: Nauka, 1977, p. 626.
Azatyan, V.V., Bakulev, V.I., Kalkanov, V.A., Romanenko, N.T., Kharlamov, A.V., and Shavard, A.A., RF Patent 1835139, 1992.
Azatyan, V.V., Tsepnye reaktsii v gorenii, vzryve i detonatsii gazov (Chain Reactions in Combustion, Explosion and Detonation of Gases), Moscow: Izd. Ross. Akad. Nauk, 2020.
Kondrat’ev, V.N. and Nikitin, E.E., Khimicheskie protsessy v gazakh (Chemical Processes in Gases), Moscow: Nauka, 1981.
Azatyan, V.V., Kinet. Katal., 1976, vol. 17, no. 2, p. 533.
Azatyan, V.V. and Shavard, A.A., Izv. Akad. Nauk SSSR, Ser. Khim., 1977, vol. 42, no. 11, p. 2460.
Azatyan, V.V., Kinet. Katal., 2015, vol. 56, no. 1, p. 3.
Azatyan, V.V., Fiz. Goreniya Vzryva, 1979, vol. 15, no. 5, p. 62.
Azatyan, V.V., Dokl. Akad. Nauk, 2020, vol. 495, p. 59. https://doi.org/10.31857/S2686953520060047
Gershenzon, Yu.M., Glebova, O.N., and Azatyan, V.V., Dokl. Akad. Nauk SSSR, 1966, vol. 168, no. 4, p. 851.
Prokopenko, V.M. and Azatyan, V.V., Zh. Fiz. Khim., 2018, vol. 92, no. 1, p. 55. https://doi.org/10.7868/S004445371801020X
Korobeinichev, O.P., Rybitskaya, I.V., Shmakov, A.G., Chernov, A.A., Bolshova, T.A., and Shvartsberg, V.M., in Proceedings of the Combustion Institute, 2009, vol. 32, p. 2591.
Azatyan, V.V., Vartanyan, A.A., Kalkanov, V.A., and Shavard, A.A., Khim. Fiz., 1989, vol. 8, no. 11, p. 1290.
Explosion, Bol’shaya Rossiiskaya entsiklopediya (Large Russian Encylopedia), Moscow: Bol’shaya Rossiiskaya entsiklopediya, 2006, vol. 5, p. 242.
Azatyan, V.V., Naboko, I.M., Petukhov, V.A., Gusev, P., Fortov, V.G., and Solntsev, O.V., Dokl. Ross. Akad. Nauk, 2004, vol. 394, no. 1, p. 61.
Lewis, B. and Friauf, J.B., J. Am. Chem. Soc., 1930, vol. 52, p. 3905.
Semenov, N.N., Tsepnye reaktsii (Chain Reactions), Leningrad: Goskhimtekhizdat, 1934.
Zel’dovich, Ya.B., Zh. Eksp. Teor. Fiz., 1940, vol. 10, p. 524.
Zel’dovich, Ya.B., Izbrannye trudy (Selected Works), Moscow: Nauka, 1984.
Von Neumann, J., OSRD Report 549, 1942.
Doring, W., Ann. Phys., 1943, vol. 43, p. 421.
Sokolik, A.S., Samovosplamenenie, plamya i detonatsiya v gazakh (Self-Combustion, Flame and Detonation in Gases), Moscow: Izd. Akad. Nauk SSSR, 1960.
Aldushin, A.P., Prog. Astronaut. Aeronaut., 1997, vol. 173, p. 95.
Nettleton, M., Gaseous Detonations, Dordrecht: Springer, 1987.
Mitrofanov, V.V., Detonatsiya gomogennykh i geterogennykh sistem (Detonation of Homogeneous and Heterogeneous Systems), Novosibirsk: Izd. Sib. Otd. Ross. Akad. Nauk, 2003.
Azatyan, V.V., Prokopenko, V.M., Abramov, S.K., and Smirnov, N.N., Dokl. Ross. Akad. Nauk, 2017, vol. 472, no. 3, p. 295.
Gel’fand, B.E., Fiz. Goreniya Vzryva, 2002, vol. 38, no. 5, p. 101.
Azatyan, V.V., Kinet. Katal., 1996, vol. 37, no. 4, p. 512.
Azatyan, V.V., Baklanov, D.I., Gvozdeva, L.G., Logutov, Yu.P., Merzhanov, A.G., and Shcherbak, N.B., Dokl. Ross. Akad. Nauk, 2001, vol. 376, no. 1, p. 55.
Azatyan, V.V., Abramov, S.K., Prokopenko, V.M., Ratnikov, V.I., and Tunik, Yu.V., Kinet. Katal., 2013, vol. 54, no. 5, p. 553.
Zel’dovich, Ya.B. and Kompaneets, A.S., Teoriya detonatsii (Theory of Detonation), Moscow: Gostekhizdat, 1955.
Azatyan, V.V., Alymov, M.I., Prokopenko, V.M., Abramov, S.K., and Kazanskii, V.B., Kinet. Katal., 2022, vol. 63, no. 4, p. 484.
Funding
This study was supported by the Ministry of Science of Higher Education of the Russian Federation (agreement no. 075-15-2020-806 of September 29, 2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest.
Additional information
Translated by L. Smolina
Abbreviations and notation: CCs are the chain carriers; CTE is the chain thermal explosion; SWs, shock waves; EPR, electronic paramagnetic resonance; М, the third particle that takes the recombination energy from НО2; q+, heat release rate; q–, heat removal rate; Т, temperature; W, reaction rate; \(Q\), thermal effect; S, reaction chamber surface area; V, reaction chamber volume; \({{\alpha \;}}\), heat transfer coefficient; T0, reactor wall temperature; В, О2; t, time; t0, initial moment of time; tr, reaction time; n, concentration of H (CC) atoms; n0, CC concentration at t0; \({{{{\omega }}}_{0}}\), rate of reaction (0); f and \(g\), rates of reactions (I) and (IV) at unit CC concentrations; k, rate constant of the intermolecular reaction; ko, preexponential factor of the rate constant k; k1, rate constant of the chain branching reaction (limiting stage); \(k_{1}^{{\text{o}}}\), preexponential factor of the rate constant k1; С0 and С, initial and current reagent concentrations, respectively; ЕR and ЕМ, activation energies of the reactions of the initial molecules with free atoms and with one another, respectively; Ebr, activation energy of chain branching; Е, effective activation energy; and R, gas constant.
Rights and permissions
About this article
Cite this article
Azatyan, V.V. Mechanism and Kinetic Laws of Reactions Determining Flame Propagation, Gas Explosion, and Detonation (Review). Kinet Catal 64, 221–234 (2023). https://doi.org/10.1134/S0023158423030023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158423030023