Abstract
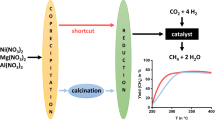
A heterogeneous catalyst, rhodium hydrotalcite (Rh-HT), was synthesized, characterized, and explored for kinetic studies for the hydrogenation of CO2 to formic acid using molecular hydrogen in an autoclave. The catalyst was efficient for the selective formation of formic acid at a moderate temperature with up to 5 catalytic cycles without any significant loss in catalytic activity. The detailed kinetic investigations were performed by determining the rate of formic acid formation as a function of time, catalyst amount, total pressure, partial pressure of CO2, partial pressure of H2, reaction volume, v/v ratio of the mixed solvent of methanol and water, agitation speed, and temperature in a wide range of variation. The rates were found to be dependent on all these parameters. The formic acid formation rate followed the first-order kinetics with respect to the partial pressures of CO2 and H2, and catalyst amount. The best reaction conditions obtained from the kinetic parametric optimisation were, 50 bar total pressure (1/1 p/p, CO2 and H2); 60°C temperature; a mixture of methanol:water solvent (5/1 v/v, 60 mL); 24 h time; and 500 rpm agitation speed to get a TON of 15840 for formic acid with no additional base. The thermodynamic performance of the heterogeneous catalyst Rh-HT was appreciably associated with highly negative entropy. The performance of the catalyst was effectively enhanced by the mixture of water and methanol as a solvent. The mechanistic routes for CO2 hydrogenation to formic acid are proposed and discussed based on the kinetic and experimental observations involving the role of the molecular effect of water used in the solvent.











Similar content being viewed by others
REFERENCES
Navarro-Jaén, S., Virginie, M., Bonin, J., Robert, M., Wojcieszak, R., and Khodakov, A.Y., Nat. Rev. Chem., 2021, vol. 5, p. 564.
Saravanan, A., Senthil Kumar, P., Dai-Viet, N.V., Jeevanantham, S., Bhuvaneswari, V., Narayanan, V.A., Yaashikaa, P.R., Swetha, S. and Reshma, B., Chem. Eng. Sci., 2021, vol. 236, p. 116515.
Osman, A.I., Hefny, M., Maksoud, M.I.A.A., Elgarahy, A.M., and Rooney, D.W., Environ. Chem. Lett., 2021, vol. 19, p. 797.
Hepburn, C., Adlen, E., Beddington, J., Carter, E.A., Fuss, S., Dowell, N.M., Minx, J.C., Smith, P., and Williams, C.K., Nature, 2019, vol. 575, p. 87.
Elgrishi, N., Chambers, M.B., Wang, X., and Fontecave, M., Chem. Soc. Rev., 2017, vol. 46, p. 761.
Perazio, A., Lowe, G., Gobetto, R., Bonin, J., and Robert, M., Coord. Chem. Rev., 2021, vol. 443, p. 214018.
Koizumi, H., Takeuchi, K., Matsumoto, K., Fukaya, N., Sato, K., Uchida, M., Matsumoto, S., Hamura, S., and Choi, J.-C., Commun. Chem., 2021, vol. 4, p. 66.
Wang, W., Wang, S., Ma, X., and Gong, J., Chem. Soc. Rev., 2011, vol. 40, p. 3703.
Gorbunov, D.N., Nenasheva, M.V., Terenina, M.V., Kardasheva, Y.S., Kardashev, S.V., Naranov, E.R., Bugaev, A.L., Soldatov, A.V., Maximov, A.L., and Karakhanov, E.A., Petrol. Chem., 2022, vol. 62, p. 1.
Siek, S., Burks, D.B., Gerlach, D.L., Liang, G., Tesh, J.M., Thompson, C.R., Qu, F., Shankwitz, J.E., Vasquez, R.M., Chambers, N., Szulczewski, G.J., Grotjahn, D.B., Webster, C.E., and Papish, E.T., Organometallics, 2017, vol. 36, p. 1091.
Bizzari, S.N. and Blagoev, M., CEH Marketing Research Report: Formic Acid. Chemical Economics Handbook, SRI Consulting, 2020. http://www.sriconsulting.com/CEH/Public/Reports/659.2000/. Accessed July 2011.
Navlani-García, M., Mori, K., Salinas-Torres, D., Kuwahara, Y., and Yamashita, H., Front. Mater., 2019, vol. 6, p. 44.
Eppinger, J. and Huang, K.-W., ACS Energy Lett., 2017, vol. 2, p. 188.
Agrawal, K., Roldan, A., Kishore, N., and Logsdail, A.J., Catal. Today, 2022, vols. 384–386, p. 197.
Jeong, B., Jeon, H., Toyoshima, R., Crumlin, E.J., Kondoh, H., Mun, B.S., and Lee, J., J. Phys. Chem. C, 2018, vol. 122, p. 2064.
Lu, C., Waste to Renewable Biohydrogen, Academic Press, 2021, vol. 1, ch. 9, p. 201.
Mao, L., Jackson, L., and Jackson, T., J. Power Sources, 2017, vol. 362, p. 39.
Navlani-García, M., Mori, K., Kuwahara, Y., and Yamashita, H., NPG Asia Mater., 2018, vol. 10, p. 277.
Zhang, Z.W., Zheng, W.T., and Jiang, Q., Int. J. Hydrogen Energy, 2012, vol. 37, p. 5090.
Liu, W., Zhao, Y.H., Li, Y., Jiang, Q., and Lavernia, E.J., J. Phys. Chem. C, 2009, vol. 113, p. 2028.
Fellay, C., Dyson, P.J., and Laurenczy, G., Angew. Chem. Int. Ed., 2008, vol. 47, p. 3966.
Rivard, E., Trudeau, M., and Zaghib, K., Materials (Basel), 2019, vol. 12, p. 1973.
Nielsen, M., Kammer, A., Cozzula, D., Junge, H., Gladiali, S., and Beller, M., Angew. Chem. Int. Ed., 2011, vol. 50, p. 9593.
Voẞ, D., Kahl, M., and Albert, J., ACS Sustain. Chem. Eng., 2020, vol. 8, p. 10444.
Valentini, F., Kozell, V., Petrucci, C., Marrocchi, A., Gu, Y., Gelman, D., and Vaccaro, L., Energy Environ. Sci., 2019, vol. 12, p. 2646.
Enthaler, S., Langermann, J.V., and Schmidt, T., Energy Environ. Sci., 2010, vol. 3, p. 1207.
Waldie, K.M., Ostericher, A.L., Reineke, M.H., Sasayama, A.F., and Kubiak, C.P., ACS Catal., 2018, vol. 8, p. 1313.
Jeletic, M.S., Hulley, E., Helm, M.L., Mock, M.T., Appel, A.M., Wiedner, E.S., and Linehan, J.C., ACS Catal., 2018, vol. 7, p. 6008.
Gassner, F. and Leiter, W., J. Chem. Soc. Chem. Commun., 1993, p. 1465.
Graf, E. and Leiter, W., J. Chem. Soc. Chem. Commun., 1992, p. 623.
Darensbourg, D.J. and Ovalles, C., J. Am. Chem. Soc., 1984, vol. 106, p. 3750.
Himeda, Y., Green Chem., 2009, vol. 11, p. 2018.
Hyde, J.R., Walsh, B., Singh, J., and Poliakoff, M., Green Chem., 2009, vol. 7, p. 357.
Fan, Li., Sakaiya, Y., and Fujimoto, K., App. Catal. A. Gen., 1999, vol. 180, p. L11.
Zhang, J., Wang, H., Li, Z., and Wang, C., Trans. Metal Chem., 1995, vol. 20, p. 118.
Oshima, K., Shinagawa, T., Nogami, Y., Manabe, R., Ogo, S., and Sekine, Y., Catal. Today, 2014, vol. 232, p. 27.
Himeda, Y., CO 2 Hydrogenation Catalysis, 2021.
Behr, A. and Nowakowski, K., Advances in Inorganic Chemistry, 2014, ch. 7, vol. 66, p. 223.
Jessop, P.G., Ikariya, T., and Noyori, R., Chem. Rev., 1995, vol. 95, pp. 259–272.
Jessop, P.G., The Handbook of Homogenous Hydrogenation, de Vries, J.G. and Elsevier, C.J., Eds., Weinheim: Wiley-VCH, 2007, vol. 1, p. 490.
Preti, D., Squarcialupi, S., and Fachinetti, G., Angew. Chem. Int. Ed., 2010, vol. 49, p. 2581.
Sun, R., Liao, Y., Bai, S.-T., Zheng, M., Zhou, C., Zhang, T., and Sels, B.F., Energy Environ. Sci., 2021, vol. 14, p. 1247.
Ye, R.-P., Ding, J., Gong, W., Argyle, M.D., Zhong, Q., Wang, Y., Russell, C.K., Xu, Z., Russell, A.G., Li, Q., Fan, M., and Yao, Y.-G., Nat. Commun., 2019, vol. 10, p. 1.
Whang, H.S., Lim, J., Choi, M.S., Lee, J., and Lee, H., BMC Chem. Eng., 2019, vol. 9, p. 1.
Maru, M.S., Patel, P., Khan, N.H., and Shukla, R.S., Curr. Catal., 2020, vol. 9, p. 59.
Maru, M.S., Ram, S., Shukla, R.S., and Noor-ul, H.K., Mol. Catal., 2018, vol. 446, p. 23.
Patel, P., Nandi, S., Maru, M.S., Kureshy, R.I., and Noor-ul, H.K., J. CO 2 Util., 2018, vol. 25, p. 310.
Maru, M.S., Ram, S., Adwani, J.H., and Shukla, R.S., ChemistrySelect, 2017, vol. 2, p. 3823.
Sudheesh, N., Chaturvedi, A.K., and Shukla, R.S., Appl. Catal. A. Gen., 2011, vol. 409, p. 99.
Sudheesh, N., Sharma, S.K., Shukla, R.S., and Jasra, R.V., J. Mol. Catal. A. Chem., 2010, vol. 316, p. 23.
Sudheesh, N., Sharma, S.K., Shukla, R.S., and Jasra, R.V., J. Mol. Catal. A. Chem., 2008, vol. 296, p. 61.
Srivastava, V.K., Sharma, S.K., Shukla, R.S., Subrahmanyam, N., and Jasra, R.V., Ind. Eng. Chem. Res., 2005, vol. 44, p. 1764.
Maru, M.S., Ram, S., Noor-ul, H.K., and Shukla, R.S., Mater. Adv., 2021, vol. 2, p. 5443.
Srivastava, V., Catal. Lett., 2021, vol. 151, pp. 3704–3720.
Mitchell, C.E., Terranova, U., Alshibane, I., Morgan, D.J., Davies, T.E., He, Q., Hargreaves, J.S.J., Sankar, M., and de Leeuw, N.H., New J. Chem., 2019, vol. 43, pp. 13985–13997.
Liu, Q., Yang, X., Li, L., Miao, S., Li, Y., Li, Y., Wang, X., Huang, Y., and Zhang, T., Nat. Commun., 2017, vol. 8, p. 1407.
Sharma, S.K., Parikh, P.A., and Jasra, R.V., J. Mol. Catal. A: Chem., 2010, vol. 317, p. 27.
Sharma, S.K., Srivastava, V.K., Shukla, R.S., Parikh, P.A., and Jasra, R.V., New J. Chem., 2007, vol. 31, p. 277.
Jasra, R.V., Srivastava, V.K., Shukla, R.S., Bajaj, H.C., and Bhatt, S.D., US Patent 7294745 B2, 2007.
Sharma, D. and Sakthivel, A., J. Nanosci. Nanotechnol., 2018, vol. 18, p. 381.
Motokura, K., Hashimoto, N., Hara, T., Mitsudome, T., Mizugaki, T., Jitsukawa, K., and Kaneda, K., Green Chem., 2011, vol. 13, p. 2416.
Gunasekar, G.H., Park, K., Jung, K.-D., and Yoon, S., Inorg. Chem. Front., 2016, vol. 3, p. 882.
Wiyantoko, B., Kurniawati, P., Purbaningtias, T.E., and Fatimah, Is., Procedia Chem., 2015, vol. 17, p. 21.
Jing, C., Zhang, Q., Liu, X., Chen, Y., Wang, X., Xia, L., Zeng, H., Zhang, D.W.W., and Dong, F., RSC Adv., 2019, vol. 9, p. 9604.
Constantino, V.R.L. and Pinnavaia, T.J., J. Inorg. Chem., 1995, vol. 34, no. 4, p. 883.
Kim, Y., Kang, S., Kang, D., Lee, K.R., Song, C.K., Sung, J., Kim, J.S., Lee, H., Park, J., and Yi, J., Angew. Chem. Int. Ed., 2021, vol. 60, p. 25411.
Samal, A.K., Zhu, H., Harb, M., Sangaru, S.S., Anjum, D.H., Hedhili, M.N., Saih, Y., and Basset, J.-M., Catal. Sci. Technol., 2017, vol. 7, p. 581.
Gallaher, G., Goodwin, J.G., Huang, C.S., and Houalla, M., J. Catal., 1991, vol. 127, p. 719.
Fierro, J.L.G., Palacios, J.M., and Tomas, F., Surf. Interface Anal., 1988, vol. 13, p. 25.
László, B., Baán, K., Varga, E., Oszkó, A., Erdohelyi, A., Kónya, Z., and Kiss, J., Appl. Catal. B: Environ., 2016, vol. 199, p. 473.
Wang, Y., Nie, Y.G., Pan, J.S., Pan, L.K., Sun, Z., Wang, L.L., and Sun, C.Q., Phys. Chem. Chem. Phys., 2010, vol. 12, p. 2177.
Iordan, A., Zaki, M.I., Kappenstein, C., and Geron, C., Phys. Chem. Chem. Phys., 2003, vol. 5, p. 1708.
Zhang, T., Zhao, B., Chen, Q., Peng, X., Yang, D., and Qiu, F., Appl. Biol. Chem., 2019, vol. 62, p. 12.
Wu, L.Q., Li, Y.C., Li, S.Q., Li, Z.Z., Tang, G.D., Qi, W.H., Xue, L.C., Ge, X.S. and Ding, L.L., AIP Adv., 2015, vol. 5, p. 097210.
Lucrédio, A.F., Filho, G.T., and Assaf, E.M., Appl. Surf. Sci., 2009, vol. 255, p. 5851.
Stephen, H. and Stephen, T., Solubilities of Inorganic and Organic Compounds, New York: Pergamon Press, 1963, vol. 1, pt. 1–2.
Sudheesh, N., Parmar, J.N., and Shukla, R.S., Appl. Catal. A., 2012, vol. 124, p. 415.
Ansari, M.B., Shukla, R.S., Mo, Y.-H., and Park, S.-E., Chem. Eng. J. Adv., 2021, vol. 6, p. 100102.
Tsai, J.C. and Nicholas, K.M., J. Am. Chem. Soc., 1992, vol. 114, p. 5117.
Funding
CSMCRI communication no. IMC 03, CSIR-CSMCRI–56/2022. The authors thank the Council of Scientific and Industrial Research (CSIR), New Delhi, India for the financial support through the Network Project on Clean Coal Technology (Tap Coal, CSC 0102) and the Analytical Division and Central Instrumentation Facility of CSIR Central Salt and Marine Chemicals Research Institute, Bhavnagar, for providing instrumental analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Abbreviations and notation: HT, hydrotalcite; Rh-HT, rhodium hydrotalcite; RRh-HT, recycled rhodium hydrotalcite catalyst; RWGS, reverse water gas shift; PXRD, powder X-ray diffraction; FT-IR, Fourier-transform infrared spectroscopy; BET, Brunauer–Emmett–Teller method; XPS, X-ray photoelectron spectroscopy; ICP, inductive coupled plasma; HPLC, high-performance liquid chromatography; FE-SEM/EDX, field emission scanning electron microscopy with energy-dispersive X-ray spectroscopy; TON, number of moles of product formed per mole of rhodium in catalyst.
Rights and permissions
About this article
Cite this article
Maru, M., Ram, S. & Shukla, R.S. Kinetic Investigation on Selective CO2 Hydrogenation to Formic Acid over Rhodium Hydrotalcite (Rh-HT) Catalyst. Kinet Catal 64, 276–293 (2023). https://doi.org/10.1134/S0023158423030060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158423030060