Abstract
The expansion of the vascular cambium cylinder in the stem of woody plants has been modeled many times, using different approaches and focusing on contributions of different cell events (cell divisions, intrusive cell growth and symplastic cell growth). Although there are many case studies in the literature, a universal model is still lacking. Therefore, the aim of this study is to estimate the quantitative changes in the contribution of symplastic growth of a single cambial cell (a sector of the cambial circumference) to the expansion of the vascular cambium cylinder, as the stem increases in girth. The proposed calculations, using the number π, and considering the actual dimensions of cambial cells, show (a) that the average symplastic increase per one initial cell in the circumferential direction decreases exponentially with the enlargement of cambial circumference, and (b) that the significant difference in the magnitude of symplastic increment of a single initial in the radial and circumferential directions increases proportionally to the increase in the circumference of the cambial cylinder. The proposed mathematical formula helps to understand the general rules that govern the gradual increase of the vascular cambium cylinder during wood production and would further facilitate the description/modeling of stem growth and formation of wood structural patterns.
Similar content being viewed by others
Introduction
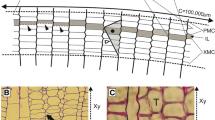
The vascular cambium is a lateral meristem responsible for the production of secondary vascular tissues of woody plants, i.e., secondary xylem (wood) and secondary phloem. Information on the development, structure and growth of this meristem has been reviewed by many authors (e.g., Esau, 1965; Bannan, 1968; Murmanis, 1970; Zimmermann & Brown, 1971; Catesson, 1974; Fahn, 1990; Larson, 1994; Iqbal, 1990, 1994, 1995; Lachaud, 1999; Spicer & Groover, 2010; Tomescu & Groover, 2019; Shi et al., 2019; Miodek et al., 2021; Wilczek-Ponce et al., 2021). Within a stem, the vascular cambium has the shape of a cylinder composed of cambial initials that do not form a continuous layer but are rather arranged as an irregular network of cells forming “the initial surface” (Włoch & Połap, 1994; Włoch et al., 2013). The two types of the cambial initials, viz. fusiform initials and ray initials, may form two general patterns in tangential view, i.e., storied (when fusiform initials occur in horizontal tiers) or non-storied (when fusiform initials do not form horizontal tiers but terminate at varied levels of height) (Iqbal, 1990, 1994; Larson, 1994).
The cell events that occur in the vascular cambium are the periclinal and anticlinal divisions, plus the symplastic growth and intrusive growth. While periclinal divisions are tangent to the surface of cambium and often unequal (Barlow, 2005), anticlinal divisions can be longitudinal (radial) (Krawczyszyn, 1977; Cumbie, 1984), oblique (Bannan, 1968; Hejnowicz, 1968) and occasionally transverse (Hejnowicz, 1963). Symplastic growth is a coordinated growth of cells in the cylinder that behaves as a common framework, wherein the contiguous cells grow in unison and therefore the existing intercellular contacts do not change (Priestley, 1930; Iqbal, 1990; Larson, 1994). In contrast, intrusive growth of cambial initials, normally confined to cell apices, leads to change in mutual cell contacts and hence in cell rearrangement, which is later reflected in the structure of deposited secondary vascular tissues as, for instance, the spiral or interlocked wood grain (Harris, 1989; Włoch et al. 2002; Kojs et al. 2004a, b).
Over the years, the expansion of a cambial cylinder has been modeled taking into account a different concept of contribution of these cell events. Classic works, referring to the species with storied cambium, attributed the expansion in cambial circumference to increase in the number of cambial cells as a result of radial anticlinal divisions (Butterfield, 1972; Cumbie, 1984) and increment of their circumferential diameter (i.e., symplastic growth) (Butterfield, 1973). In contrast, the expansion of the cambium in species with non-storied structure was considered to be associated with increase in the number of cambial cells due to anticlinal division, an apical intrusive growth of cells between radial walls of sister initials following the oblique anticlinal divisions, and the complementary cell elimination (cell loss) from the layer of initials, the latter two being considered as the two independent cambial events (Bannan, 1950; Hejnowicz & Brański, 1966; Srivastava, 1973; Zagórska-Marek & Little, 1986; Larson, 1994; Barlow et al., 2002). However, later Kojs et al. (2004b), Jura et al. (2006) and Wilczek et al. (2011) have suggested that intrusive growth and cell elimination are two faces of the same process, and elimination is not a separate cambial event. Also, the findings, referring to the cambia of species like Laburnum anagyroides, Lonchocarpus sericeus, Picea abies, Pinus sylvestris, Robinia pseudoacacia, Tilia cordata and Wisteria floribunda, have produced structural and quantitative evidence to establish that the cambial initials grow intrusively between the periclinal walls of the adjacent initial and its immediate derivative, and that merely the symplastic growth of cambial cells is sufficient to attain the required increase in the circumference of the cambial cylinder, irrespective of the cambium being storied or non-storied (Włoch and Połap 1994; Włoch et al. 2002, 2009, 2013; Kojs et al. 2004a, b; Jura et al. 2006; Karczewska et al. 2009; Wilczek et al. 2018; Miodek et al. 2021, 2022; Wilczek-Ponce et al. 2021).
Although there are many case studies available in the literature, there is a lack of a universal model of growth of the cambial cylinder. Given this, the present study aims at estimating the quantitative changes of symplastic cell growth that occur in the vascular cambium cylinder, as the stem increases in girth. The proposed mathematical formula, together with the actual dimensions of the cambial cells, allows for estimating the increase of the cambial cylinder at the level of a single cell (a sector of the circumference) and helps to understand the general rules that govern the gradual increase of the vascular cambium cylinder during wood production.
Materials and Methods
List of Mathematical Symbols
C – Circumference of cambial cylinder (C = 2πr).
C/N – Circumferential dimension of initial cell (part of cambial circumference occupied by one initial).
ΔC –Increase in cambial circumference by symplastic growth.
ΔC1 –Increase in cambial circumference following the deposition of one layer of cells (ΔC1 = 2πΔr1).
N – Number of cambial initial cells on the cambial circumference.
r – Radius of cambial cylinder.
Δr – Change in radius of cambial cylinder.
Δr1 –Change in radius of cambial cylinder following the deposition of one layer of cells.
Assumptions in Mathematical Calculations
The following assumptions have been taken into consideration regarding the actual dimensions of cambial initials and the cambial growth:
-
(a)
the vascular cambium is a uniseriate layer of initial cells (Schmid, 1976), able to grow (symplastically and intrusively) and divide (anticlinally and periclinally);
-
(b)
ray cells and fusiform cells have similar width in transverse section (Catesson, 1974, 1984);
-
(c)
the average width of one initial cell is approximately 20 μm and thickness 10 μm (Esau, 1965; Evert, 2006; Miodek et al., 2021; Wilczek-Ponce et al., 2021);
-
(d)
each cambial cell is a sector of a cambial cylinder circumference that increases in a coordinated (symplastic) way (Miodek et al., 2021, Wilczek-Ponce et al., 2021);
-
(e)
in a cylinder-shaped cambium, the periclinal walls of the cell have to be curved (convex), hence it is necessary to make use of π in the model of the growth of the whole cambium cylinder as well as in a very small sector of the circle, equivalent to the width of one radial row of cells;
-
(f)
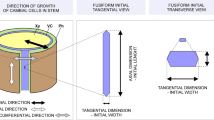
symplastic growth of cambial cell occurs largely in the radial direction, slightly in the circumferential direction, and not at all in the axial direction (Wilczek et al., 2018; Miodek et al., 2021);
-
(g)
symplastic growth is calculated in the transverse plane of the vascular cambium cylinder;
-
(h)
the average increase in the length of radius of the vascular cambium cylinder after deposition of one layer of cells on the xylem side (Δr1) is 10 μm (Miodek et al., 2021);
-
(i)
the increase in cambial circumference (ΔC1) due to the deposition of one layer of cells on xylem side is 62.8 μm (ΔC1 = 2πΔr1);
-
(j)
cambial initials grow intrusively between periclinal walls and this growth is associated only with cells readjustment, having no role in the increase of the cambial circumference (Wilczek-Ponce et al., 2021; Miodek et al., 2021);
-
(k)
radial growth of stem generates mechanical stresses on the vascular cambium, which are relaxed by cambial cell readjustment, involving the additional anticlinal divisions and the intrusive growth of initials (Włoch et al., 2013).
Terminology
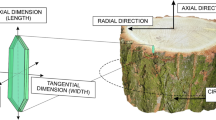
Since the surface of the cambial cylinder is curved, and the tangent plane touches a limited surface area of the cylinder and can be described only on a small anatomical section (Fig. 1), the use of the term ‘tangential’ to describe the enlargement of the curved (circumferential) surface of the vascular cambium cylinder is not appropriate. It is better to replace the term ‘tangential’ with ‘circumferential’ while describing a dimension that has been calculated using the number π for every row of the cambial cells.
Results and Discussion
The General Rules of the Increase of Cambial Cylinder
In our model, the increase in the circumference of the cambium is linked to the coordinated symplastic growth of all the initial cells present on the circumference of the cambium. The growth of each initial cell is proportional to the increase of the circumference (gradual growth; Table 1). It means that the initial cells adjust their width to the change in length of the radius (Δr) of the vascular cambium cylinder, keeping the average width of the initial cells commensurate to their number by additional anticlinal divisions. For instance, a 10 μm increase in the radius (Δr1), due to deposition of one layer of cells on the inner side of the cambial cylinder, corresponds to 62.8 μm increase in its circumference (ΔC1 = 2πΔr1). As the mean width of the cambial cells is 20 μm, the growth of circumference of the cambial cylinder is maintained by complementary anticlinical divisions (limited divisions, Table 1). The total number of anticlinal divisions taken place does not match with the magnitude of growth of the cambium circumference. Usually the widest cells divide, but often the frequency of anticlinal divisions is much higher than required by the growing cambial circumference, as observed especially in the non-storied cambia (Hejnowicz & Brański, 1966; Hejnowicz, 1968; Zagórska-Marek & Little, 1986). This excess number of anticlinal divisions and the intrusive growth of the sister cells produced are associated with the rearrangement of the cambium cells which lose their status of being the initials because of having been pushed out of the layer of initials, and hence no longer contribute to the increase of surface of the cambium cylinder (Kojs et al. 2004a, b). Włoch et al. (2013) have presented diagrams simulating the cross-sectional and tangential views of cambial cells during the cell rearrangement. The basic differences in contribution of cambial cell events to the overall form of the storied and non-storied cambia are presented in Table 1.
Any increase in the radius of the cambial cylinder (Δr) is dependent on the frequency of periclinal divisions of the cambial initials and their immediate xylem mother cells that may produce a variable number of derivatives (Larson, 1994). The ratio of radial increase (Δr, i.e., symplastic growth of derivative cells in radial direction) to circumferential increase (ΔC) of a vascular cambium is a constant value (that is Δr/ΔC), regardless of whether Δr is equal to 10 μm (Δr1 in our model, and in that of Wilczek-Ponce et al., 2021), or 25 μm (Miodek et al., 2021), or any other value.
Circumferential and Radial Symplastic Increase of a Single Cambial cell
Symplastic growth in circumferential direction calculated per one initial cell is insignificant in comparison to its much greater extent in the radial direction (Table 2; Fig. 2). The value of ΔC1/N (increment in the circumference of a single initial cell) varies depending on the size of the cambium circumference (C), i.e., the number of initial cells on the circumference (N), assuming that the width of the cells has a constant value (here 20 μm). A difference in the magnitude of the symplastic increment of a single initial in the radial and circumferential directions increases proportionally to the increase in the circumference of the cambium cylinder Δr/(ΔC/N). For instance, when the number of cells (N) in a cambial circumference (C) of 10 cm is 5,000, and Δr1 = 10 μm, then Δr1/(ΔC1/N) = 10/(62.8/5,000) = 796.18, but for a cylinder of circumference of 1 m, and N = 50,000, this ratio would be 7,961.78 (Table 2; Fig. 2a). Thus, Δr/(ΔC/N) will keep increasing as the radius, and hence the circumference of the cambium, and hence the total number of cells in the circumference increases. In other words, as the cambial circumference grows bigger and bigger, the ratio of symplastic cell growth in radial direction versus circumferential direction correspondingly increases. To be more specific, the larger the cambial circumference (number of cells, N), the smaller is the required extent of symplastic growth per one initial cell in the circumferential direction, or the lesser is the required number of anticlinal division of initials.
The chart of a the relationship between the size of the circumference of the cambium cylinder (C) and the extent of symplastic growth of a single cambial initial in circumferential direction after deposition of one layer of cells on the xylem side (ΔC1/N), and b the relationship between the increase of cambium circumference (C) and the ratio of radial to circumferential increase of a cambial initial cell by symplastic growth {Δr1/(ΔC1/N)}
The Proposed Model Versus Previous Models of Cambium Increase
The classic models typically associated the increase of cambial circumference with the number of anticlinal divisions (e.g., Butterfield, 1972, 1973), and also other cambial events like the intrusive growth (Hejnowicz & Brański, 1966), depending on the cambium type, storied or non-storied. Calculations of the expansion of cambial circumference based on the number of anticlinal divisions referred only to storied cambia. For instance, Butterfield (1972), indicated that the percentage of the fusiform cambial initials that need to divide anticlinally in order to fulfill the requirement of the expanding cambial circumference in storied cambium of Aeschynomene hispida decreases per millimeter of radial growth. Later, Butterfield (1973) noticed that apart from cambial cell number by radial anticlinal divisions, the diameter of cells also increased, as the cambium expanded. In general, our model is in line with the results of Butterfield, where the increase in the number of cambial cells refers to the increase in circumference of the vascular cambium cylinder, and the sister cells expand proportionally in circumferential direction. Taking into account the current state of knowledge, the Buttefield’s approach could be applicable also to the non-storied cambium.
Barlow et al. (2002) compared a disc-shaped thallus of alga Coleochaete orbicularis, resembling the transverse section of the growing cambium, with the growth of the non-storied cambium of hybrid aspen (Populus tremula x P. tremuloides). Although such comparison is accurate, and in agreement with our model, especially because the vascular cambium as a cylinder-shaped tissue does not grow in the axial direction, and therefore its growth has to be described on the basis of transverse sections of the cambial cylinder, Barlow et al. (2002) described the increase of the cambium circumference taking into account the supposed contribution of intrusive growth of fusiform cells also. According to the current state of knowledge, the intrusive growth contributes to the rearrangement of cambial cells, and not to the enlargement of cambial cylinder (e.g., Włoch et al., 2013; Wilczek-Ponce et al., 2021). Barlow et al. (2002) also suggested that the rate of growth of the enlarged initial cell needs not to be the same in each direction, and recently Miodek et al. (2021) have calculated that in a cambial cylinder of 1 m circumference, a cambial cell grows symplastically nearly 8,000 times faster in the radial direction than in the circumferential direction. Our general assumptions for calculations regarding the contribution of cambial events to the increase of the cambial cylinder are in line with the calculations of symplastic growth of the cambial initials by Miodek et al. (2021) and Wilczek-Ponce et al. (2021). However, our model shows a general tendency of contribution of symplastic cell growth calculated per one cambial initial in a cylinder of different radii.
The proposed model emphasizes upon the fact that symplastic growth of cambial cells is sufficient to meet the requirement for the enlargement of a cambial cylinder during the stem growth. On the other hand, the intrusive growth of the cambial cells is associated with cell rearrangement, leading to the formation of the wood structural patterns such as the spiral, wavy or interlocked grains (e.g. Hejnowicz, 1971, 1990; Hejnowicz & Romberger, 1973; Hejnowicz & Zagórska-Marek, 1974; Harris, 1989; Włoch et al., 2002). The intensity of intrusive growth does not depend on the size of the cambial circumference and the speed of radial growth. It is usually enormous and rapid (Table 1), both in storied and non-storied cambium type (Włoch et al. 2002; Kojs et al. 2004a). It only affects the rearrangement of cambial cells and, consequently, the relaxation of mechanical shear stresses in the stem. These stresses are generated by the internal and external (environmental) factors like wind or precipitation (Kojs & Rusin, 2011; Miodek et al., 2021). Recent research on spiral grain of wood seem to be focused more on the mathematical description of such growth patterns, considering them to result from the influence of external factors, thus ignoring the significant role of the vascular cambium in this context (e.g., Ekevad, 2005; Leelavanichkul & Cherkaev, 2004).
Conclusion
Knowledge about the functioning and development of the vascular cambium is essential to understand the process of wood formation. The contribution of different cell events (frequency of periclinal/anticlinal divisions and intensity of intrusive/symplastic cell growth) to the growth of the vascular cambium cylinder has been interpreted variously in different models of the cambial growth. Our simple mathematical model, where each initial cell is a small segment of the cambium circumference, takes into account the current state of knowledge on the cambial events, and predicts the contribution of symplastic growth of cambial initials to the increase of a cambial cylinder with different diameter. The model is universal and clarifies the general rules that govern the gradual increase of the vascular cambium cylinder during wood production from a storied or non-storied cambium. A clear understanding of the role of symplastic growth in the enlargement of the cambial cylinder, and of intrusive growth solely in the readjustment of cells in the cambial surface may further facilitate the descriptions/modeling of stem growth and wood structural patterns.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Bannan, M.W. (1950). The frequency of anticlinal divisions in fusiform cambial cells of Chamaecyparis. American Journal of Botany 37, 511–519.
Bannan, M.W. (1968). Anticlinal divisions and the organization of the conifer cambium. Botanical Gazette 129, 107–113.
Barlow, P.W. (2005). From cambium to early cell differentiation within the secondary vascular system. In N.M. Holbrook & M.A. Zwieniecki (Eds.), Vascular transport in plants (pp. 279–306). Elsevier Academic Press, Amsterdam.
Barlow, P.W., Brain, P., Powers, S.J. (2002). Estimation of directional division frequencies of vascular cambium and in marginal meristematic cells of plants. Cell Proliferation 35, 49–68.
Butterfield, B.G. (1972). Developmental changes in the vascular cambium of Aeschynomene hispida Willd. New Zealand Journal of Botany 10, 373–386.
Butterfield, B.G. (1973). Variation in the size of fusiform cambial initials and vessel members in Hoheria angustifolia Raoul. New Zealand Journal of Botany 11, 391–410.
Catesson, A.M. (1974). Cambial cells. In A.W. Robards (Ed.), Dynamic aspects of plant ultrastructure (pp. 358–390). Mc Graw–Hill, London, Toronto.
Catesson, A.M., (1984). Cambial dynamics. Annales des Sciences Naturelles 6, 23–44.
Cumbie, B.G. (1984). Origin and development of the vascular cambium in Aeschynomene virginica. Bulletin of Torrey Botanical Club 111, 42–50.
Ekevad, M. (2005). Twist of wood studs: dependence on spiral grain gradient. Journal of Wood Science 51, 455–461.
Esau, K., (1965). Plant anatomy. John Wiley and Sons, New York.
Evert, R.F. (2006). Esau’s Plant anatomy; meristems, cells, and tissues of the plant body: their structure, function, and development. 3rd ed. John Wiley & Sons Inc, Hoboken, New Jersey.
Fahn, A. (1990). Plant anatomy. 4th ed. Pergamon Press Ltd, Oxford, London.
Harris, J.M. (1989). Spiral grain and wave phenomena in wood formation. Springer–Verlag, Berlin.
Hejnowicz, Z. (1963). Intrusive growth, transverse and pseudotransverse divisions in fusiform initials of wounded cambium in Larix europea. Acta Societatis Botanicum Poloniae 32, 493–503.
Hejnowicz, Z. (1968). The structural mechanism involved in the changes of grain in timber. Acta Societatis Botanicum Poloniae 37, 347–365.
Hejnowicz, Z. (1971). Upward movement of the domain pattern in the cambium producing wavy grain in Picea excelsa. Acta Societatis Botanicum Poloniae 40, 499–512.
Hejnowicz, Z. (1990). Phenomena of orientation in the cambium. In M. Iqbal (Ed.), The vascular cambium (pp. 127–13). Research Studies Press, Taunton, England.
Hejnowicz, Z., Brański, S. (1966). Quantitative analysis of cambium growth in Thuja. Acta Societatis Botanicum Poloniae 35, 395–400.
Hejnowicz, Z., Romberger, J.A. (1973). Migrating cambial domains and the origin of wavy grain in xylem of broad–leaved trees. American Journal of Botany 60, 209–222.
Hejnowicz, Z., Zagórska–Marek, B. (1974). Mechanism of changes in grain inclination in wood produced by storeyed cambium. Acta Societatis Botanicum Poloniae 43, 381–398.
Iqbal, M. (1990). The vascular cambium. John Wiley & Sons, Chichester, UK.
Iqbal, M. (1994). Structural and operational specializations of the vascular cambium of seed plants. In M. Iqbal (Ed.), Growth patterns in vascular plants (pp. 211–271). Dioscordies Press, Portland, Oregon.
Iqbal, M. (1995). Structure and behaviour of vascular cambium and the mechanism and control of cambial growth. In M. Iqbal (Ed.), The cambial derivatives (pp. 1–67). Gebrüder Borntraeger, Stuttgart, Germany.
Jura, J., Kojs, P., Iqbal, M., Szymanowska–Pułka, J., Włoch, W. (2006). Apical intrusive growth of cambial fusiform initial along the tangential walls of adjacent fusiform initials: evidence for a new concept. Australian Journal of Botany 54, 493–504.
Karczewska, D., Karczewski, J., Włoch, W., Jura-Morawiec, J., Kojs, P., Iqbal, M., Krawczyszyn, J. (2009). Mathematical modelling of intrusive growth of fusiform initials in relation to radial growth and expanding cambial circumference in Pinus sylvestris L. Acta Biotheoretica 57,331–348.
Kojs, P., Rusin, T. (2011). Diurnal strains in plants. In J. Gliński, J. Horabik, & J. Lipiec (Eds.), Encyclopedia of Agrophysics (pp. 220–224). Springer, Berlin.
Kojs, P., Włoch, W., Rusin, A. (2004a). Rearrangement of cells in storied cambium of Lonchocarpus sericeus (Poir.) DC. connected with formation of interlocked in the xylem. Tress 18, 136–144.
Kojs, P., Włoch, W., Iqbal, M., Rusin, A., Jura, J. (2004b). Readjustments of cambial initials in Wisteria floribunda (Willd.) DC. for development of storeyed structure. New Phytologist 163, 287–297.
Krawczyszyn, J. (1977). The transition from non–storeyed to storeyed cambium in Fraxinus excelsior. 1. The occurrence of radial anticlinal divisions. Canadian Journal of Botany 55, 3034–3041.
Lachaud, S., Catesson, A.M., Bonnemain, J.L. (1999). Structure and function of the vascular cambium. Life Sciences 322, 633–650.
Larson, P.R. (1994). The vascular cambium: Development and structure. Springer–Verlag, Berlin.
Leelavanichkul, S. & Cherkaev, A. (2004). Why the grain in tree trunks spirals: a mechanical perspective. Structural and Multidisciplinary Optimization 28, 127–135.
Miodek, A., Włoch, W., Iqbal, M., Gizińska, A., Kojs, P. (2021). Controversy over the mode of growth of cambial cylinder. The Botanical Review 87, 243–257.
Miodek, A., Gizińska, A., Włoch, W., Kojs, P. (2022). Intrusive growth of initials does not affect cambial circumference in Robinia pseudoacacia. Scientific Reports 12, 7428.
Murmanis, L. (1970). Locating the initial in the vascular cambium of Pinus strobus L. by electron microscopy. Wood Science and Technology 4, 1–14.
Priestley, J.H. (1930). Studies in the physiology of cambial activity. II. The concept of sliding growth. New Phytologist 29, 96–104.
Schmid, R. (1976). The elusive cambium – another terminological contribution. IAWA Bulletin 4, 51–59.
Shi, D., Lebovka, I., López-Salmerón, V., Sanchez, P., Greb, T. (2019). Bifacial cambium stem cells generate xylem and phloem during radial plant growth. Development 146, 171355.
Spicer, R., Groover, A. (2010). Evolution and development of vascular cambia and secondary growth. New Phytologist 186, 577–592.
Srivastava, L.M. (1973). Cambial activity in trees. Arnoldia 33, 46–66.
Tomescu, A., Groover, A. (2019). Mosaic modularity: an updated perspective and research agenda for the evolution of vascular cambial growth. New Phytologist 222, 1719–1735.
Wilczek, A., Jura-Morawiec, J., Kojs, P., Iqbal, M, Włoch, W. (2011). Correlation of intrusive growth of cambial initials to rearrangement of rays in vascular cambium. IAWA Jounal 32(3), 313–332.
Wilczek, A., Iqbal, M., Włoch, W., Klisz, M. (2018). Geometric analysis of intrusive growth of wood fibers in Robinia pseudoacacia. IAWA Journal 39,191–208.
Wilczek-Ponce, A., Włoch, W., Iqbal, M. (2021). How do trees grow in girth? Controversy on the role of cellular events in the vascular cambium. Acta Biotheoretica 69, 643–670.
Włoch, W. & Połap, E. (1994). The intrusive growth of initial cells in re-arrangement of cells in cambium of Tilia cordata Mill. Acta Societatis Botanicum Poloniae 63,109–116.
Włoch, W., Mazur, E., Bełtowski, M. (2002). Formation of spiral grain in the wood of Pinus sylvestris L. Trees 16, 306–312.
Włoch, W., Jura-Morawiec, J., Kojs, P., Iqbal, M., Krawczyszyn, J. (2009). Does intrusive growth of fusiform initials really contribute to circumferential growth of vascular cambium? Botany 87, 154–163.
Włoch, W., Wilczek, A., Jura-Morawiec, J., Kojs, P., Iqbal, M. (2013). Modelling for rearrangement of fusiform initials during radial growth of the vascular cambium in Pinus sylvestris L. Trees 27, 879–893.
Zagórska-Marek, B. & Little, C.H.A. (1986). Control of fusiform initial orientation in the vascular cambium of Abies balsamea stems by indol-3-ylacetic acid. Canadian Journal of Botany 64, 1120–1128.
Zimmermann, M.H. & Brown, C.L. (1971). Trees: structure and function. Springer, New York.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
WW and JJM designed research and wrote the initial draft. MI critically revised and improved the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Competing Interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Włoch, W., Iqbal, M. & Jura-Morawiec, J. Calculating the Growth of Vascular Cambium in Woody Plants as the Cylindrical Surface. Bot. Rev. 89, 237–249 (2023). https://doi.org/10.1007/s12229-023-09291-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-023-09291-z