Abstract
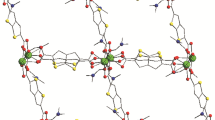
1,2-Bis[(2,6-diisopropyl-4-diethylmalonophenyl)imino]acenaphthene (Dem-Bian) with zinc chloride forms complex [(Dem-Bian)ZnCl2] (I). The reaction of complex I with n-BuLi proceeds with the deprotonation of the malonate fragments and gives 1D coordination polymer [ZnCl2(Dem-Bian)Li(DME)2]n (II). The reaction of [(Dem-Bian)CuCl] with n-BuLi affords 1D polymer [(Dem-Bian)Li2(DME)2]n (III). Compounds I–III are characterized by elemental analysis and IR spectroscopy. Derivatives I and II are characterized by 1Н NMR spectroscopy. The crystal structures of compounds II and III are determined by X-ray diffraction (XRD). Their thermal stability is studied by thermogravimetric analysis.






Similar content being viewed by others
REFERENCES
Bernauer, J., Pölker, J., and von Wangelin, A.J., Chem. Cat. Chem., 2022, vol. 14, no. 1, р. e202101182.
Marreiros, J., Diaz-Couce, M., Ferreira, M.J., et al., Inorg. Chim. Acta, 2019, vol. 486, p. 274.
Beltrani, M., Carfagna, C., Milani, B., et al., Adv. Synth. Catal., 2016, vol. 358, no. 20, p. 3244.
Moskalev, M.V., Skatova, A.A., Chudakova, V.A., et al., Russ. Chem. Bull., 2015, vol. 64, no. 12, p. 2830.
Moskalev, M.V., Yakub, A.M., Morozov, A.G., et al., Eur. J. Org. Chem., 2015, vol. 2015, no. 26, p. 5781.
Rumble, S.L., Page, M.J., Field, L.D., et al., Eur. J. Inorg. Chem., 2012, vol. 2012, no. 13, p. 2226.
Li, L., Lopes, P.S., Rosa, V., et al., Dalton Trans., 2012, vol. 41, no. 17, p. 5144.
Fedushkin, I.L., Moskalev, M.V., Lukoyanov, A.N., et al., Chem.-Eur. J., 2012, vol. 18, no. 36, p. 11264.
Fedushkin, I.L., Nikipelov, A.S., Morozov, A.G., et al., Chem.-Eur. J., 2012, vol. 18, no. 1, p. 255.
Viganó, M., Ragaini, F., Buonomenna, M.G., et al., ChemCatChem, 2010, vol. 2, no. 9, p. 1150.
Alonso, J.C., Neves, P., Pires da Silva, M.J., et al., Organometallics, 2007, vol. 26, no. 23, p. 5548.
Gottumukkala, A.L., Teichert, J.F., Heijnen, D., et al., J. Org. Chem., 2011, vol. 76, no. 9, p. 3498.
de Fremont, P., Clavier, H., Rosa, V., et al., Organometallics, 2011, vol. 30, no. 8, p. 2241.
Yu, X., Zhu, F., Bu, D., et al., RSC Adv., 2017, vol. 7, no. 25, p. 15321.
Sandl, S., Maier, T.M., van Leest, N.P., et al., ACS Catal., 2019, vol. 9, no. 8, p. 7596.
Soshnikov, I.E., Bryliakov, K.P., Antonov, A.A., et al., Dalton Trans., 2019, vol. 48, no. 23, p. 7974.
Wang, F., Tanaka, R., Li, Q., et al., Organometallics, 2018, vol. 37, no. 9, p. 1358.
Liu, Z.W.Q., Solan, G.A., and Sun, W.-H., Coord. Chem. Rev., 2017, vol. 350, p. 68.
Guo, L., Liu, W., and Chen, C., Mater. Chem. Front., 2017, vol. 1, no. 12, p. 2487.
Small, B.L., Rios, R., Fernandez, E.R., et al., Organometallics, 2010, vol. 29, no. 24, p. 6723.
Popeney, C.S. and Guan, Z., Macromolecules, 2010, vol. 43, no. 9, p. 4091.
Miyamura, Y., Kinbara, K., Yamamoto, Y., et al., J. Am. Chem. Soc., 2010, vol. 132, no. 10, p. 3292.
Romain, C., Rosa, V., Fliedel, C., et al., Dalton Trans., 2012, vol. 41, no. 12, p. 3377.
Liu, J., Li, Y., Li, Y., et al., J. Appl. Pol. Sci., 2008, vol. 109, no. 2, p. 700.
Wang, F. and Chen, C., Polym. Chem., 2019, vol. 10, no. 19, p. 2354.
Brown, L.A., Wekesa, F.S., Unruh, D.K., et al., J. Pol. Sci. A, 2017, vol. 55, no. 17, p. 2824.
Kazarina, O.V., Gourlaouen, C., Karmazin, L., et al., Dalton Trans., 2018, vol. 47, no. 39, p. 13800.
Мorozov, A.G., Markelova, E.S., Fedyushkin, I.L., et al., Russ. J. Appl. Chem., 2018, vol. 1, no. 6, p. 1044.
Fedushkin, I.L., Morozov, A.G., Chudakova, V.A., et al., Eur. J. Inorg. Chem., 2009, no. 33, p. 4995.
Bazyakina, N.L., Makarov, V.M., Ketkov, S.Yu., et al., Inorg. Chem., 2021, vol. 60, p. 3238.
Koptseva, T.S., Bazyakina, N.L., Moskalev, M.V., et al., Eur. J. Inorg. Chem., 2021, vol. 60, p. 3238.
Bazyakina, N.L., Moskalev, M.V., Cherkasov, A.V., et al., CrystEngComm, 2022, vol. 24, p. 2297.
Koptseva, T.S., Bazyakina, N.L., Rumyantcev, R.V., et al., Mendeleev Commun., 2022, vol. 32, p. 780.
Bazyakina, N.L., Makarov, V.M., Moskalev, M.V., et al., Mendeleev Commun., 2022, vol. 32, p. 759.
Su, J., Yuan, S., Li, J., et al., Chem.-Eur. J., 2021, vol. 27, p. 622.
Bigdeli, F., Lollar, C.T., Morsali, A., et al., Angew. Chem., Int. Ed. Engl., 2020, vol. 59, p. 4652.
Calbo, J., Golomb, M.J., and Walsh, A., J. Mater. Chem. A, 2019, vol. 7, p. 16571.
Su, J., Yuan, S., Li, J., et al., Chem.-Eur. J., 2021, vol. 27, p. 622.
Li, B., Zhao, Y.M., Kirchon, A., et al., J. Am. Chem. Soc., 2019, vol. 141, p. 6822.
Sokolov, V.G., Moskalev, M.V., Koptseva, T.S., et al., Russ. Chem. Bull., 2021, vol. 69, no. 1, p. 125.
Bazhina, E.S., Aleksandrov, G.G., Kiskin, M.A., et al., Russ. J. Coord. Chem., 2020, vol. 46, no. 2, p. 89. https://doi.org/10.1134/S1070328420020025
Bazhina, E.S., Shmelev, M.A., Babeshkin, K.A., et al., Russ. Chem. Bull., 2021, vol. 70, no. 11, p. 2130.
Blinou, D.O., Zorina-Tikhonova, E.N., Voronina, Yu.K., et al., Russ. J. Coord. Chem., 2022, vol. 48, no. 8, p. 487. https://doi.org/10.1134/S1070328422080012
APEX3. Bruker Molecular Analysis Research Tool. Version 2018.7-2, Madison: Bruker AXS Inc., 2018.
Data Collection, Reduction and Correction Program. CrysAlisPro 1.171.40.67a – Software Package, Rigaku OD, 2019.
SAINT. Data Reduction and Correction Program. Version 8.38A, Madison (WI): Bruker AXS Inc., 2017.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., and Stalke, D., J. Appl. Crystallogr., 2015, vol. 48, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Adv., 2015, vol. 71, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3.
Sheldrick, G.M., SHELXTL. Version 6.14. Structure Determination Software Suite, Madison (WI): Bruker AXS, 2003.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, p. 339.
Sheldrick, G.M., SADABS. Version 2016/2. Bruker/Siemens Area Detector Absorption Correction Program, Madison: Bruker AXS Inc., 2016.
SCALE3 ABSPACK: Empirical Absorption Correction. CrysAlisPro 1.171.40.67a – Software Package, Rigaku OD, 2019.
ACKNOWLEDGMENTS
This work was carried out using the equipment of the Center for Collective Use “Analytical Center of Razuvaev Institute of Organometallic Chemistry of Russian Academy of Sciences” supported by the grant “Provision of Development of Material Technical Infrastructure of Centers for Collective Use of Scientific Equipment” (unique identifier RF−2296.61321X0017, agreement no. 075-15-2021-670).
Funding
This work was supported by the Russian Science Foundation, project no. 19-13-00336-П.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Bazyakina, N.L., Sokolov, V.G., Moskalev, M.V. et al. Coordination Polymers of Lithium Based on 1,2-Bis[(2,6-diisopropyl-4-diethylmalonophenyl)imino]acenaphthene. Russ J Coord Chem 49, 397–406 (2023). https://doi.org/10.1134/S1070328422600620
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328422600620