Abstract
Purpose
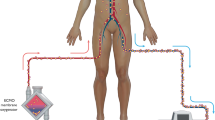
Adequate dosing of antimicrobials is critical to properly treat infections and limit development of resistance and adverse effects. Limited guidance exist for antimicrobial dosing adjustments in patients requiring extracorporeal membrane oxygenation (ECMO) therapy, particularly in the pediatric population. A systematic review was conducted to delineate the pharmacokinetics (PK) and pharmacodynamics (PD) of antimicrobials in critically ill neonates and children requiring ECMO therapy.
Methods
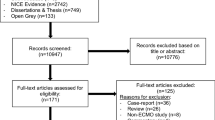
Medline, EMBASE, Global Health and All EBM Reviews databases were queried. Grey literature was examined. All clinical studies reporting PK/PD parameters of antimicrobials in critically ill pediatric patients treated with ECMO were included, except for case reports and congress abstracts. Two independent reviewers applied the inclusion and exclusion criteria. Reviewers were then paired to independently extract data and evaluate the methodological quality of studies using the ROBINS-I tool and the compliance with ClinPK reporting guidelines. Patient and study characteristics, key PK/PD findings, details of ECMO circuits and co-treatments were summarized qualitatively. Broad dosing recommendations were formulated based on the available data for specific antimicrobials.
Results
Twenty-nine clinical studies were included; most were observational and uncontrolled. Patient characteristics and co-treatments were often missing. The effect of ECMO on PK/PD parameters of antimicrobials varied depending on the drugs and population studied. It was only possible to formulate dosing recommendations for a few antimicrobials given the paucity of data, its overall low quality and heterogeneity in reporting.
Conclusion
Limited data exists on the PK/PD of antimicrobials during ECMO therapy in the pediatric population. Rigorously designed population PK studies are required to establish empiric dosing guidelines for antimicrobials in patients requiring this therapeutic modality. The use of therapeutic drug monitoring for antimicrobials in pediatric patients on ECMO should be encouraged to optimize dosing.
Trial Registry
PROSPERO registration number: CRD42018099992 (Registered: July 24th 2018).


Similar content being viewed by others
References
Ha MA, Sieg AC. Evaluation of altered drug pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation. Pharmacotherapy. 2017;37:221–35. https://doi.org/10.1002/phar.1882.
Gijsen M, Vlasselaers D, Spriet I, Allegaert K. Pharmacokinetics of antibiotics in pediatric intensive care: fostering variability to attain precision medicine. Antibiotics. 2021;10:1182. https://doi.org/10.3390/antibiotics10101182.
Shekar K, Roberts JA, McDonald CI, Ghassabian S, Anstey C, Wallis SC, Mullany DV, Fung YL, Fraser JF. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care. 2015;19:164. https://doi.org/10.1186/s13054-015-0891-z.
Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–16. https://doi.org/10.1007/s00134-010-2041-z.
Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12:277–81. https://doi.org/10.1097/PCC.0b013e3181e28894.
Cashen K, Reeder R, Dalton HJ, Berg RA, Shanley TP, Newth CJL, Pollack MM, Wessel D, Carcillo J, Harrison R, Dean JM, Tamburro R, Meert KL. Acquired infection during neonatal and pediatric extracorporeal membrane oxygenation. Perfusion. 2018;33:472–82. https://doi.org/10.1177/0267659118766436.
Ayyildiz P, Kasar T, Ozturk E, Yildiz O, Ozturk S, Ergul Y, Haydin S, Guzeltas A. The evaluation of nosocomial infections in pediatric patients with extracorporeal membrane oxygenation support. Braz J Cardiovasc Surg. 2017;32:468–74. https://doi.org/10.21470/1678-9741-2017-0072.
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. https://doi.org/10.1097/01.CCM.0000217961.75225.E9.
Duceppe MA, Kanji S, Do AT, Ruo N, Cavayas AY, Albert M, Robert-Halabi M, Zavalkoff S, Dupont P, Samoukovic G, Williamson DR. Pharmacokinetics of commonly used antimicrobials in critically ill adults during extracorporeal membrane oxygenation: a systematic review. Drugs. 2021;81:1307–29. https://doi.org/10.1007/s40265-021-01557-3.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. https://doi.org/10.1186/2046-4053-4-1.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919. https://doi.org/10.1136/bmj.i4919.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020. https://doi.org/10.1002/jrsm.1411.
Kanji S, Hayes M, Ling A, Shamseer L, Chant C, Edwards DJ, Edwards S, Ensom MHH, Foster DR, Hardy B, Kiser TH, la Porte C, Roberts JA, Shulman R, Walker S, Zelenitsky S, Moher D. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK statement. Clin Pharmacokinet. 2015;54:783–95. https://doi.org/10.1007/s40262-015-0236-8.
Ahsman MJ, Wildschut ED, Tibboel D, Mathot RA. Pharmacokinetics of cefotaxime and desacetylcefotaxime in infants during extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 2010;54:1734–41. https://doi.org/10.1128/AAC.01696-09.
Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996;40:1139–42. https://doi.org/10.1128/AAC.40.5.1139.
Autmizguine J, Hornik CP, Benjamin DK, Brouwer KL, Hupp SR, Cohen-Wolkowiez M, Watt KM. Pharmacokinetics and safety of micafungin in infants supported with extracorporeal membrane oxygenation. Pediatr Infect Dis J. 2016;35:1204–10. https://doi.org/10.1097/inf.0000000000001268.
Cohen P, Collart L, Prober CG, Fischer AF, Blaschke TF. Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J. 1990;9:562–6. https://doi.org/10.1097/00006454-199008000-00007.
Dodge WF, Jelliffe RW, Zwischenberger JB, Bellanger RA, Hokanson JA, Snodgrass WR. Population pharmacokinetic models: effect of explicit versus assumed constant serum concentration assay error patterns upon parameter values of gentamicin in infants on and off extracorporeal membrane oxygenation. Ther Drug Monit. 1994;16:552–9.
Hoie EB, Swigart SA, Leuschen MP, Willett LD, Bolam DL, Goodrich PD, Bussey ME, Nelson RM. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. 1990;9:711–5.
Munzenberger PJ, Massoud N. Pharmacokinetics of gentamicin in neonatal patients supported with extracorporeal membrane oxygenation. ASAIO Trans. 1991;37:16–8. https://doi.org/10.1097/00002480-199101000-00006.
Southgate WM, DiPiro JT, Robertson AF. Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1989;33:817–9. https://doi.org/10.1128/aac.33.6.817.
Thibault C, Moorthy GS, Vedar C, Naim MY, DiLiberto MA, Zuppa AF. Pharmacokinetics of cefepime in children on extracorporeal membrane oxygenation: external model validation, model improvement and dose optimization. Pediatr Infect Dis J. 2022;41:217–23. https://doi.org/10.1097/INF.0000000000003371.
Wang Y, Chen W, Huang Y, Wang G, Li Z, Yan G, Chen C, Lu G. Optimized dosing regimens of meropenem in septic children receiving extracorporeal life support. Front Pharmacol. 2021;12: 699191. https://doi.org/10.3389/fphar.2021.699191.
Watt KM, Benjamin DK Jr, Cheifetz IM, Moorthy G, Wade KC, Smith PB, Brouwer KL, Capparelli EV, Cohen-Wolkowiez M. Pharmacokinetics and safety of fluconazole in young infants supported with extracorporeal membrane oxygenation. Pediatr Infect Dis J. 2012;31:1042–7. https://doi.org/10.1097/INF.0b013e31825d3091.
Zuppa AF, Zane NR, Moorthy G, Dalton HJ, Abraham A, Reeder RW, Carcillo JA, Yates AR, Meert KL, Berg RA, Sapru A, Mourani P, Notterman DA, Dean JM, Gastonguay MR. A population pharmacokinetic analysis to study the effect of extracorporeal membrane oxygenation on cefepime disposition in children. Pediatr Crit Care Med. 2019;20:62–70. https://doi.org/10.1097/PCC.0000000000001786.
Zylbersztajn B, Parker S, Navea D, Izquierdo G, Ortiz P, Torres JP, Fajardo C, Diaz R, Valverde C, Roberts J. Population pharmacokinetics of vancomycin and meropenem in pediatric extracorporeal membrane oxygenation support. Front Pharmacol. 2021;12: 709332. https://doi.org/10.3389/fphar.2021.709332.
An SH, Lee EM, Kim JY, Gwak HS. Vancomycin pharmacokinetics in critically ill neonates receiving extracorporeal membrane oxygenation. Eur J Hosp Pharm. 2020;27:e25–9. https://doi.org/10.1136/ejhpharm-2018-001720.
Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1992;12:28–32.
Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1998;18:1082–6.
Cies JJ, Moore WS, Nichols K, Knoderer CA, Carella DM, Chopra A. Population pharmacokinetics and pharmacodynamic target attainment of vancomycin in neonates on extracorporeal life support. Pediatr Crit Care Med. 2017;18:977–85. https://doi.org/10.1097/pcc.0000000000001250.
Lonabaugh KP, Lunsford KJ, Fang GY, Kaufman DA, Addison SD, Buck ML. Vancomycin dosing in pediatric extracorporeal membrane xxygenation: potential impacts of new technologies. J Pediatr Pharmacol Ther. 2017;22:358–63. https://doi.org/10.5863/1551-6776-22.5.358.
Moffett BS, Morris J, Galati M, Munoz FM, Arikan AA. Population pharmacokinetic analysis of gentamicin in pediatric extracorporeal membrane oxygenation. Ther Drug Monit. 2018;40:581–8. https://doi.org/10.1097/ftd.0000000000000547.
Moffett BS, Morris J, Galati M, Munoz F, Arikan AA. Population pharmacokinetics of vancomycin in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2018;19:973–80. https://doi.org/10.1097/PCC.0000000000001682.
Mulla H. An investigation into the effects of extracorporeal membrane oxygenation on pharmacokinetics. [Thesis]. Leicester: De Montfort University; 2003.
Saito J, Shoji K, Oho Y, Kato H, Matsumoto S, Aoki S, Nakamura H, Ogawa T, Hasegawa M, Yamatani A, Miyairi I. Population pharmacokinetics and pharmacodynamics of meropenem in critically ill pediatric patients. Antimicrob Agents Chemother. 2021;65:e01909-e1920. https://doi.org/10.1128/AAC.01909-20.
Suwa J, Sakurai R, Horikoshi Y, Ishihara Y. Pharmacokinetics of vancomycin in children undergoing combined extracorporeal membrane oxygenation and continuous venovenous hemodiafiltration therapy [Japanese]. Jpn J Chemotherapy. 2019;67:38–43.
Zylbersztajn BL, Izquierdo G, Santana RC, Fajardo C, Torres JP, Cordero J, Valverde C. Therapeutic drug monitoring of vancomycin in pediatric patients with extracorporeal membrane oxygenation support. J Pediatr Pharmacol Ther. 2018;23:305–10. https://doi.org/10.5863/1551-6776-23.4.305.
Di Nardo M, Cairoli S, Goffredo BM, Stoppa F, D’Argenio P, Corsetti T, Ranieri VM. Therapeutic drug monitoring for meropenem after the extracorporeal membrane oxygenation circuit change in children: is it necessary? Minerva Anestesiol. 2016;82:1018–9.
Lindsay CA, Bawdon R, Quigley R. Clearance of ticarcillin-clavulanic acid by continuous venovenous hemofiltration in three critically ill children, two with and one without concomitant extracorporeal membrane oxygenation. Pharmacotherapy. 1996;16:458–62.
Wildschut ED, de Hoog M, Ahsman MJ, Tibboel D, Osterhaus AD, Fraaij PL. Plasma concentrations of oseltamivir and oseltamivir carboxylate in critically ill children on extracorporeal membrane oxygenation support. PLoS ONE. 2010;5: e10938. https://doi.org/10.1371/journal.pone.0010938.
Zylbersztajn B, Izquierdo CG, Navea MD, Torres TJP, Valverde GC. Concentraciones plasmáticas de piperacilina/tazobactam en pacientes pediátricos críticos sometidos a ECMO. Análisis preliminar. [Spanish]. Rev Chilena Infectol. 2020;37:216–8. https://doi.org/10.4067/s0716-10182020000300216.
Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol. 2005;60:265–75. https://doi.org/10.1111/j.1365-2125.2005.02432.x.
Yalcin N, Sürmelioğlu N, Allegaert K. Population pharmacokinetics in critically ill neonates and infants undergoing extracorporeal membrane oxygenation: a literature review. BMJ Paediatr Open. 2022;6: e001512. https://doi.org/10.1136/bmjpo-2022-001512.
Sutiman N, Koh JC, Watt K, Hornik C, Murphy B, Chan YH, Lee JH. Pharmacokinetics alterations in critically ill pediatric patients on extracorporeal membrane oxygenation: a systematic review. Front Pediatr. 2020;8:260. https://doi.org/10.3389/fped.2020.00260.
Fernandez E, Perez R, Hernandez A, Tejada P, Arteta M, Ramos JT. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics. 2011;3:53–72. https://doi.org/10.3390/pharmaceutics3010053.
Acknowledgements
We thank M. Patrice Dupont, librarian at the Université de Montréal, for his expertise and help with the literature search strategies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
Marc-Alexandre Duceppe: No conflict of interest to declare. Salmaan Kanji: No conflict of interest to declare. Anh Thu Do: No conflict of interest to declare. Ni Ruo: No conflict of interest to declare. Yiorgos Alexandros Cavayas: No conflict of interest to declare. Martin Albert: No conflict of interest to declare. Maxime Robert-Halabi: No conflict of interest to declare. Samara Zavalkoff: No conflict of interest to declare. Laura Benichou: No conflict of interest to declare. Gordan Samoukovic: No conflict of interest to declare. David Williamson: No conflict of interest to declare.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
Code availability
Not applicable.
Author contributions
MAD: This author had the idea for the article, led the research design, created and performed the search strategy, screened for citations, prepiloted the case report forms, evaluated selected citations and participated in the writing and revision of the manuscript. SK: This author helped in the research design, evaluated selected citations and participated in the writing and revision of the manuscript. ATD: This author helped in the research design, evaluated selected citations and participated in the writing and revision of the manuscript. NR: This author helped in the research design, evaluated selected citations and participated in the writing and revision of the manuscript. YAC: This author helped in the research design, evaluated selected citations and participated in the writing and revision of the manuscript. MA: This author helped in the research design, evaluated selected citations and participated in the writing and revision of the manuscript. MRH: This author helped in the research design, evaluated selected citations and participated in the writing and revision of the manuscript. SZ: This author helped in the research design, evaluated selected citations and participated in the writing and revision of the manuscript. LB: This author screened for citations, evaluated selected citations and participated in the writing and revision of the manuscript. GS: This author had the idea for the article, helped in the research design, evaluated selected citations and participated in the writing and revision of the manuscript. DW: This author helped in the research design, screened for citations, prepiloted the case report forms, evaluated selected citations and participated in the writing and revision of the manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duceppe, MA., Kanji, S., Do, A.T. et al. Pharmacokinetics of Commonly Used Antimicrobials in Critically Ill Pediatric Patients During Extracorporeal Membrane Oxygenation: A Systematic Review. Pediatr Drugs 25, 515–535 (2023). https://doi.org/10.1007/s40272-023-00582-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00582-x