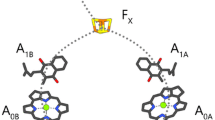
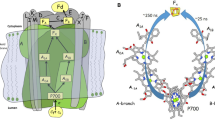
Abstract
Time-resolved step-scan FTIR difference spectroscopy at 77 K has been used to study photosystem I (PSI) from Synechocystis sp. PCC 6803 with four high-potential, 1,4–naphthoquinones (NQs) incorporated into the A1 binding site. The incorporated quinones are 2–chloro–NQ (2ClNQ), 2–bromo–NQ (2BrNQ), 2,3–dichloro–NQ (Cl2NQ), and 2,3–dibromo–NQ (Br2NQ). For completeness 2-methyl-NQ (2MNQ) was also incorporated and studied. Previously, PSI with the same quinones incorporated were studied in the, so-called, anion spectral region between 1550 and 1400 cm−1 (Agarwala et al. in Biochim Biophys Acta 1864(1):148918, 2023). Here we focus on spectra in the previously unexplored 1400–1200 cm−1 spectral region. In this region several bands are identified and assigned to the neutral state of the incorporated quinones. This is important as identification of neutral state quinone bands in the regular 1700–1600 cm−1 region has proven difficult in the past. For neutral PhQ in PSI a broad, intense band appears at ~ 1300 cm−1. For the symmetric di-substituted NQs (Cl2NQ/Br2NQ) a single intense neutral state band is found at ~ 1280/1269 cm−1, respectively. For both mono-substituted NQs, 2ClNQ and 2BrNQ, however, two neutral state bands are observed at ~ 1280 and ~ 1250 cm−1, respectively. These observations from time-resolved spectra agree well with conclusions drawn from absorption spectra of the NQs in THF, which are also presented here. Density functional theory based vibrational frequency calculations were undertaken allowing an identification of the normal modes associated with the neutral state quinone bands.







Similar content being viewed by others
Abbreviations
- Cl2NQ:
-
2,3–Dichloro–NQ
- Br2NQ:
-
2,3–Dibromo–NQ
- 2ClNQ:
-
2–Chloro–NQ
- 2BrNQ:
-
2–Bromo–NQ
- 2MNQ:
-
2–Methyl–NQ
- C=O:
-
Carbonyl
- Chl a :
-
Chlorophyll a
- DAS:
-
Decay associated spectrum
- DDS:
-
Double difference spectrum
- DFT:
-
Density functional theory
- DS:
-
Difference spectra/spectrum/spectroscopy
- ET:
-
Electron transfer
- FTIR:
-
Fourier transform infrared
- H–bond:
-
Hydrogen bond
- NQ:
-
1,4–Naphthoquinone
- PhQ:
-
Phylloquinone (2–methyl–3–phytyl–NQ)
- PQ:
-
Plastoquinone–9
- DMNQ:
-
2,3–Dimethyl–NQ
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- RC:
-
Reaction center
- S6803:
-
Synechocystis Sp. PCC 6803
- THF:
-
Tetrahydrofuran
- TRSS:
-
Time–resolved step–scan
- WT:
-
Wild type
References
Agalarov R, Brettel K (2003) Temperature dependence of biphasic forward electron transfer from the phylloquinone(s) A1 in photosystem I: only the slower phase is activated. Biochim Biophys Acta 1604:7–12
Agarwala N, Ranke D, Rohani HG (2019) Calculated and experimental infrared spectra of substituted naphthoquinones. Front Sci Technol Eng Math 3:71–80
Agarwala N, Makita H, Hastings G (2023) Time-resolved FTIR difference spectroscopy for the study of photosystem I with high potential naphthoquinones incorporated into the A1 binding site. Biochim Biophys Acta 1864:148918
Antoshvili M, Caspy I, Hippler M, Nelson N (2018) Structure and function of photosystem I in Cyanidioschyzon merolae. Photosynth Res. https://doi.org/10.1007/s11120-018-0501-4
Arnoux P, Morosinotto T, Saga G, Bassi R, Pignol D (2009) A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 21:2036–2044
Ben-Shem A, Frolow F, Nelson NJN (2003) Crystal structure of plant photosystem I. Nature 426:630–635
Breton J (2006) FTIR studies of the primary electron donor, P700. In: Golbeck J (ed) Photosystem I: The Light Driven Plastocyanin: Ferredoxin Oxidoreductase. Advances in Photosynthesis and Respiration, vol 24. Springer, Dordrecht, pp 271–289
Breton J, Nabedryk E (1996) Protein-quinone interactions in the bacterial photosynthetic reaction center: Light-induced FTIR difference spectroscopy of the quinone vibrations. Biochem Biophys Acta 1275:84–90
Breton J, Burie J-R, Boullais C, Berger G, Nabedryk E (1994) Binding sites of quinones in photosynthetic bacterial reaction centers investigated by light-induced FTIR difference spectroscopy: binding of chainless symmetrical quinones to the QA site of Rhodobacter sphaeroides. Biochemistry 33:12405–12415
Brettel K (1997) Electron transfer and arrangement of the redox cofactors in photosystem I. Biochem Biophys Acta 1318:322–373
Busch A, Hippler M (2011) The structure and function of eukaryotic photosystem I. Biochim Biophys Acta Bioenerg 1807(864):877
Byrdin M, Santabarbara S, Gu FF, Fairclough WV, Heathcote P, Redding K, Rappaport F (2006) Assignment of a kinetic component to electron transfer between iron-sulfur clusters F-X and F-A/B of Photosystem I. Biochim Biophys Acta 1757:1529–1538
Fromme P, Grotjohann I (2006) Structural analysis of cyanobacterial photosystem I. In: Golbeck JH (ed) Photosystem I: The Light Driven Plastocyanin: Ferredoxin Oxidoreductase, vol 24. Springer, Dordrecht, pp 47–69
Fromme P, Jordan P, Krauss N (2001) Structure of photosystem I. Biochim Biophys Acta 1507:5–31
Golbeck J (1992) Structure and function of photosystem I. Annu Rev Plant Physiol Plant Mol Biol 43:293–324
Golbeck JH (2006) Photosystem I the light-driven plastocyanin: ferredoxin oxidoreductase vol 24. Advances in Photosynthesis and Respiration. Springer
Golbeck J, Bryant D (1991) Photosystem I. Current topics in bioenergetics, vol 16. Academic Press, New York, pp 83–175
Hastings G (2015) Vibrational spectroscopy of photosystem I. Biochim Biophys Acta 1847:55–68
Hastings G, Bandaranayake KMP, Carrion E (2008) Time-resolved FTIR difference spectroscopy in combination with specific isotope labeling for the study of A(1), the secondary electron acceptor in photosystem 1. Biophys J 94:4383–4392
Hellwig P (2015) Infrared spectroscopic markers of quinones in proteins from the respiratory chain. Biochim Biophys Acta 1847:126–133
Itoh S, Iwaki M, Ikegami I (2001) Modification of photosystem I reaction center by the extraction and exchange of chlorophylls and quinones. Biochim Biophys Acta 1507:115–138
Johnson TW et al (2001) Recruitment of a foreign quinone into the A1 site of photosystem I: in vivo replacement of plastoquinone-9 by media-supplemented naphtoquinones in phylloquinone biosynthetic pathway mutants of Synechocystis sp. PCC 6803. J Biol Chem 276:39512–39521
Joliot P, Joliot A (1999) In vivo analysis of the electron transfer within Photosystem I: are the two phylloquinones involved? Biochem 38:11130–11136
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 angstrom resolution. Nature 411:909–917
Kotting C, Gerwert K (2005) Proteins in action monitored by time-resolved FTIR spectroscopy. ChemPhysChem 6:881–888
Lorenz-Fonfria VA (2020) Infrared difference spectroscopy of proteins: from bands to bonds. Chem Rev 120:3466–3576
Makita H, Hastings G (2015) Directionality of electron transfer in cyanobacterial photosystem I at 298 and 77 K. Febs Lett 589:1412–1417
Makita H, Hastings G (2016a) Modeling electron transfer in photosystem I. Biochim Biophys Acta 1857:723–733
Makita H, Hastings G (2016b) Time-resolved visible and infrared absorption spectroscopy data obtained using photosystem I particles with non-native quinones incorporated into the A1 binding site. Data Brief 7:1463–1468
Makita H, Hastings G (2017) Inverted region electron transfer as a mechanism for enhancing photosynthetic solar energy conversion efficiency. Proc Nat Acad Sci USA 114:9267–9272
Makita H, Hastings G (2018) Time-resolved step-scan FTIR difference spectroscopy for the study of photosystem I with different benzoquinones incorporated into the A1 binding site. Biochim Biophys Acta 1859:1199–1206
Makita H, Hastings G (2020) Time-resolved FTIR difference spectroscopy for the study of quinones in the A1 binding site in photosystem I: identification of neutral state quinone bands. Biochim Biophys Acta 1861:148173
Makita H, Rohani L, Zhao N, Hastings G (2017) Quinones in the A1 binding site in photosystem I studied using time-resolved FTIR difference spectroscopy. Biochim Biophys Acta 1858:804–813
Mezzetti A, Leibl W (2016) Time-resolved infrared spectroscopy in the study of photosynthetic systems. Photosynth Res. https://doi.org/10.1007/s11120-016-0305-3
Mula S et al (2012) Incorporation of a high potential quinone reveals that electron transfer in Photosystem I becomes highly asymmetric at low temperature. Photoch Photobio Sci 11:946–956
Nelson N, Ben-Shem A (2004) The complex architecture of oxygenic photosynthesis. Nat Rev Mol Cell Biol 5:971–982
Nelson N, Yocum CF (2006) Structure and function of photosystems I and II. Annu Rev Plant Biol 57:521–565
Pushkar YN, Golbeck JH, Stehlik D, Zimmermann H (2004) Asymmetric Hydrogen-bonding of the quinone cofactor in photosystem I probed by 13C-labeled naphthoquinones. J Phys Chem B 108:9439–9448
Redding K, van der Est A (2006) The Directionality of electron transport in photosystem I. In: Golbeck J (ed) Photosystem I: the light driven plastocyanin: ferredoxin oxidoreductase. Springer, Dordrecht, pp 413–437
Rohani L, Hastings G (2021) Assessment of the orientation and conformation of pigments in protein binding sites from infrared difference spectra. Biochim Biophys Acta 1862:148366
Santabarbara S, Kuprov I, Fairclough WV, Purton S, Hore PJ, Heathcote P, Evans MCW (2005) Bidirectional electron transfer in photosystem I: determination of two distances between P(700)(+) and A(1)(-) in spin-correlated radical pairs. Biochemistry 44:2119–2128
Schlodder E, Falkenberg K, Gergeleit M, Brettel K (1998) Temperature dependence of forward and reverse electron transfer from A1-, the reduced secondary electron acceptor in photosystem I. Biochem 37:9466–9476
Srinivasan N, Golbeck JH (2009) Protein-cofactor interactions in bioenergetic complexes: the role of the A(1A) and A(1B) phylloquinones in photosystem I. Biochim Biophys Acta 1787:1057–1088
van der Est A (2006) Electron Transfer involving phylloquinone in photosystem I. In: Golbeck J (ed) Photosystem I: The Light Driven Plastocyanin: Ferredoxin Oxidoreductase. Advances in Photosynthesis and Respiration, vol 24. Springer, Dordrecht, pp 387–411
van der Est A et al (2010) Incorporation of 2,3-disubstituted-1,4-naphthoquinones into the A1 binding site of photosystem I studied by EPR and ENDOR spectroscopy. Appl Magn Reson 37:65–83
Walker D (1993) Energy, plants and man, 2nd ed., with amendments. University Science Books, Brighton, East Sussex
Zybailov B et al (2000) Recruitment of a foreign quinone into the A1 site of photosystem I: II. Structural and functional characterization of phylloquinone biosynthetic pathway mutants by electron paramagnetic resonance and electron-nuclear double resonance spectroscopy. J Biol Chem 275:8531–8539
Acknowledgements
This material is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Award Number DE−SC−0017937 to GH. The statements made herein are solely the responsibility of the authors. NA acknowledges a fellowship from the Molecular Basis of Disease Program at Georgia State University.
Author information
Authors and Affiliations
Contributions
NA: Methodology, formal analysis, data curation, writing—original draft. GH: conceptualization, formal analysis, resources, writing—review & editing, supervision, funding acquisition, project administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agarwala, N., Hastings, G. Time-resolved FTIR difference spectroscopy for the study of photosystem I with high potential naphthoquinones incorporated into the A1 binding site 2: Identification of neutral state quinone bands. Photosynth Res 158, 1–11 (2023). https://doi.org/10.1007/s11120-023-01036-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-023-01036-8