Abstract
Background
Age-related macular degeneration (AMD) is one of the major causes of vision loss. Early AMD needs to be taken seriously, but the causal effects of lipid biomarkers on early AMD remain unclear.
Methods
In this study, two-sample Mendelian randomization (MR) analysis was performed to systematically assess the causal relationships between seven serum lipid biomarkers (apolipoprotein A (ApoA), apolipoprotein B (ApoB), total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL-C), direct low-density lipoprotein cholesterol (LDL-C), lipoprotein A [Lp(a)], and triglycerides (TG)) and risk of early AMD. In total, 14,034 cases and 91,214 controls of European ancestry were included in the analysis (number of SNPs = 11,304,110).
Results
MR estimates revealed that a higher HDL-C level is strongly associated with increased risk of early AMD (OR = 1.25, 95% CI: 1.15–1.35, P = 2.61 × 10−8). In addition, level of ApoA is also positively associated with risk of early AMD (OR = 2.04, 95% CI: 1.50–2.77, P = 6.27 × 10−6). Conversely, higher levels of TG significantly decrease the risk of early AMD (OR = 0.77, 95% CI: 0.71–0.84, P = 5.02 × 10−10). Sensitivity analyses further supported these associations. Moreover, multivariable MR analyses, adjusted for the effects of correlated lipid biomarkers, yielded similar results.
Conclusion
This study identifies causal relationships between elevated circulating HDL-C/ApoA levels and increased risk of early AMD, in addition to finding that TG specifically reduces the risk of early AMD. These findings contribute to a better understanding of the role of lipid metabolism in drusen formation, particularly in early AMD development.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is one of the major causes of irreversible visual impairment and central vision loss in individuals over the age of 50 [1]. The prevalence of AMD corresponds to approximately 8.7% of the global population and has been predicted to reach around 200 million by 2020 and nearly 288 million by 2040 [2], posing a heavy public health burden [3]. The causes of AMD are complex, involving environmental and genetic factors that influence susceptibility to its development [1]. The characteristic lesions of AMD are drusen, which are formed by deposits of extracellular debris between the retinal pigment epithelium and Bruch’s membrane [4, 5]. Currently, the progression of AMD is classified into early, intermediate, and late stages based on the severity of fundus lesions, such as drusen size and pigmentary abnormalities [6]. The pathological hallmark of early AMD is the presence of medium-size drusen (≥ 63 µm and < 125 µm) without pigmentary abnormalities [6], which are often asymptomatic and easily ignored in the clinic. Intermediate AMD features drusen of diameter ≥ 125 µm or pigmentary abnormalities [6]. Progression to late AMD is characterized by severe central vision loss caused by either neovascular AMD (nAMD) or geographic atrophy (GA). However, there are no effective medications for the GA subtype, the most prevalent subtype of late AMD, and the anti-vascular endothelial growth factor (anti-VEGF) drugs used to treat nAMD are not curative [7]. Therefore, early screening and prompt treatment are essential to maximize the likelihood of retaining functional vision.
The mechanisms of drusen initiation and formation are not yet fully understood. Preclinical studies have identified drusen-related components, such as lipids and Osteopontin [8, 9]. Lipid metabolism has long been believed to be involved in AMD pathogenesis [10,11,12]; however, the evidence is inconsistent [13]. These inconsistent results may be due to the effects of nutrition and drugs, which are difficult to control for inpatient populations [14]. In recent years, Mendelian randomization (MR) has emerged as a research method for investigating putative causal relationships between risk factors and diseases by using genetic variants as natural experiments [15]. Compared to traditional observational studies, MR is less likely to be influenced by confounding factors or reverse causation [15, 16]. Existing MR studies have reported a causal relationship between higher levels of high-density lipoprotein cholesterol (HDL-C) and intermediate/advanced AMD risk [17,18,19]; however, a consensus has yet to be reached regarding the associations between other lipid subfractions (e.g., LDL cholesterol, and TG) and AMD. Some researchers have speculated that these findings point to a role for retina-specific mechanisms, including local lipid trafficking, in AMD pathogenesis [20, 21].
There is a paucity of up-to-date and comprehensive information focused exclusively on early AMD. Crucially, a recent meta-GWAS on early AMD included 11 sources with GWAS data and fundus photography for early AMD phenotyping, representing the largest study of early AMD study to date [22]. More importantly, this study demonstrated the shared and distinct genetics between early and advanced AMD [22]. In this study, we systematically investigated the associations between seven major serum lipid biomarkers [ApoA, ApoB, CHOL, HDL-C, LDL-C, Lp(a), and TG] and risk of early AMD using MR analysis. Elucidating these relationships will help us better understand the role of lipids in early-stage AMD and identify lipid-modifying therapeutic targets.
Results
Associations of serum lipid biomarkers with early AMD
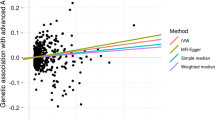
Of the seven lipid-related traits, univariable MR analysis using the IVW method found that five biomarkers were significantly associated with early AMD, including HDL-C, LDL-C, TG, ApoA, and ApoB (Table 1). MR estimates showed that higher HDL-C levels were strongly associated with increased risk of early AMD (OR = 1.25, 95% CI: 1.15–1.35, P = 2.61 × 10−8) (Figs. 1A and 2). Consistent results were found by MR analysis using three other MR methods, including weighted median (OR = 1.42, P = 4.51 × 10−9), weighted mode (OR = 1.44, P = 2.61 × 10−5), and MR Egger (OR = 1.24, P = 4.32 × 10−4). In addition, levels of ApoA were also found to be positively associated with risk of early AMD (OR = 2.04, 95% CI: 1.50–2.77, P = 6.27 × 10−6) (Figs. 1B and 2), and these estimates were supported by the other three MR methods (Table 1). These results are consistent with the high correlation between HDL-C and ApoA.
Causal relationships between serum lipid biomarkers and risk of early AMD. Causal relationships between serum HDL-C (A), ApoA (B), LDL-C (C), ApoB (D), TG (E), and CHOL (F) and risk of early AMD. The x-axis shows estimated values for serum lipid levels, and the y-axis shows estimated (log odd ratios) effects on early AMD. MR-IVW, MR-Egger, simple median, and weighted median method lines are plotted with different colors
Conversely, we found that higher LDL-C levels significantly decreased risk of early AMD (OR = 0.90, 95% CI: 0.85–0.96, P = 2.03 × 10−3) (Figs. 1C and 2). However, the estimates lost significance after Bonferroni correction in the other three MR methods (Table 1). In addition to LDL-C, higher levels of ApoB and TG were found to be negatively associated with early AMD risk (Figs. 1D–E and 2). There is strong evidence for a causal association between TG and early AMD, based on IVW (OR = 0.77, 95% CI: 0.71–0.84, P = 5.02 × 10−10), weighted median (OR = 0.78, 95% CI: 0.68–0.88, P = 8.30 × 10−5), weighted mode (OR = 0.82, 95% CI: 0.73–0.92, P = 7.30 × 10−4), and MR Egger (OR = 0.79, 95% CI: 0.69–0.89, P = 2.14 × 10−4) approaches. Estimates from the IVW and weighted mode methods indicate that a higher ApoB level is significantly associated with decreased risk of early AMD (IVW: OR = 0.52, P = 6.60 × 10−5; weighted mode: OR = 0.56, P = 1.85 × 10−3), although these results were not significant after Bonferroni correction (Table 1).
High levels of CHOL were nominally associated with decreased risk of early AMD (Figs. 1F and 2, and Table 1). No associations were observed between Lp(a) and early AMD (Supplementary Fig. 1 and Table 1). To test whether there is reciprocal causation, we estimated the effects of early AMD on lipid biomarkers. As expected, the results showed no reciprocal causation (Supplementary Table 1).
Sensitivity analysis
Using the MR-Egger intercept calculation, no evidence of directional horizontal pleiotropy was detected for any lipid-related traits (intercept was close to zero with P-value > 0.05). Results of MR-Egger intercept analysis were displayed in Table 1.
MR-PRESSO outlier-corrected tests were also performed in this study. MR analysis for all lipid-related traits showed that estimates and significance values were essentially unchanged after outlier SNPs were removed (Tables 2). These outlier SNPs were located primarily in genes that were associated with both lipid biomarkers and AMD (e.g., CETP and APOE); therefore, removing these SNPs would affect our estimated effect sizes and study power. Leave-one-out analysis was also applied to assess the robustness of our MR findings. Leave-one-out analysis was conducted by determining whether our estimates were driven or biased by single SNPs. Finally, no outliers were observed across all seven lipid-related traits and early AMD risk (Fig. S1–7).
Multivariable MR
We performed multivariable MR (MVMR)-IVW analysis to evaluate the direct effects of lipid biomarkers on early AMD by conditioning on other related traits. For the classic trio (HDL-C, LDL-C, and TG), we found strong evidence for associations between HDL-C (OR = 1.18, P = 0.0014) and TG (OR = 0.86, P = 0.0099) with the risk of early AMD. However, the association between LDL-C and early AMD was nominal (P = 0.063). Next, HDL-C was replaced with ApoA1 in the trio (ApoA, LDL-C, and TG). These results showed that ApoA (OR = 1.85, P = 0.00034) and TG (OR = 0.83, P = 0.00033) are significantly associated with risk of early AMD. For the group of ApoA, ApoB, TG, and Lp(a), the results were similar to those of univariable MR analysis (ApoA: OR = 1.58, P = 0.0060; ApoB: OR = 0.62, P = 0.0086; TG: OR = 0.83, P = 0.00017, Table 2).
Discussion
In this study, we conducted comprehensive MR analyses to investigate the causal effects of seven serum lipid biomarkers on early AMD risk. We found that higher HDL-C and ApoA levels increase the risk of early AMD, whereas LDL-C, ApoB, and TG appear to be associated with decreased risk of early AMD. The major value of this study is to fill gaps in our understanding of the causal effects of lipid biomarkers on early AMD risk. More importantly, these MR findings shed light on the different roles of lipid subfractions in early AMD and aid us in understanding the role of lipid metabolism in the initiation and formation of drusen in early stages, as well as the potential utility of blood lipid-modifying therapies in preventing and treating AMD.
Compared with previous MR studies, the direction of causal effect size was the same as the causal effects of lipids on intermediate and late AMD, for which genetically elevated HDL-C levels increase AMD risk [17,18,19]. Han et al. found that high-density HDL-C and ApoA1 levels increase the risk of intermediate, GA, CNV, and advanced AMD subtypes [19]. In our study, the effect size of HDL-C levels on early AMD was 1.25 per 1 mmol/l increase (95% CI: 1.22–1.70), which appeared smaller than the effect on intermediate AMD (OR = 1.34 per 1 mmol/l increase, 95% CI: 1.20–1.49) [19]. Notably, we identified a robust association between HDL-C and early AMD, specifically using the inverse-variance weighted, weighted median, weighted mode, and MR Egger methods. Furthermore, our study also determined that higher levels of ApoA, the major apolipoprotein in HDL-C particles, increase the risk of early AMD, with OR 2.04 (95% CI: 1.50–2.77, P = 6.27 × 10−6, Table 2). Our ever-increasing knowledge of HDL-C highlights two sides of health. On the one hand, HDL-C induces reverse cholesterol transport associated with plaque regression and has anti-inflammatory [23] and antioxidant properties [24, 25], which could strengthen endothelial function and decrease atherosclerosis risk [26]. On the other hand, the adverse effects of high HDL-C levels on AMD may partially reflect HDL dysfunction. It has become apparent that under certain conditions, such as cardiovascular disease [27], aging [28], or acute phase response, HDL-C can play pro-inflammatory and pro-oxidant roles that inhibit cholesterol efflux [29, 30]. Consequently, oxidation products, such as peroxidation lipids, gradually accumulate in the retina and Bruch’s membrane, contributing to the development of AMD. Although a 12-month pilot study by Vavvas et al. reported that high-dose atorvastatin resulted in regression of drusen deposits in 10 of 23 patients [31], the impact of lipid-modifying therapies on AMD risk is currently unknown. Our results emphasize that the potential effects of lipid-modifying drugs on AMD and related phenotypes should be further investigated.
Consistent with previous observational studies, higher TG levels reduce the risk of early AMD [10, 12]. In our study, raised TG levels were associated with decreased risk of early AMD, with OR 0.77 (95% CI: 0.71–0.84, P = 5.02 × 10−10); the effect size was robust in MR-PRESSO outlier-corrected tests. In previous observational studies, LDL-C and CHOL levels were not found to be associated with any early AMD characteristics [12]. In MR studies, the causal association between LDL-C and advanced AMD was insignificant in both Europeans and Asians [17, 18]. However, Han X. et al. found that the associations of LDL-C were primarily with GA (OR = 0.70, 95% CI: 0.59–0.83, P = 3.8 × 10−5) and intermediate AMD (OR = 0.77, 95% CI: 0.67–0.87, P = 6.5 × 10−5) but were not strong enough with nAMD [19]. Similarly, ApoB (OR = 0.76, 95% CI: 0.69–0.85) and CHOL (OR = 0.81, 95% CI: 0.70–0.94) were also associated with decreased risk of intermediate AMD [19]. In our study, the lipid biomarkers LDL-C (OR = 0.90, 95% CI: 0.85–0.96, P = 2.03 × 10−3) and ApoB (OR = 0.52, 95% CI: 0.38–0.72, P = 6.60 × 10−5) play protective roles in the development of early AMD. However, we did not find robust associations between LDL-C and ApoB with early AMD in weighted median analyses, suggesting that the relationship between LDL-C and early AMD is inconsistent. In addition, we found no evidence of an association between Lp(a) and AMD risk.
Dyslipidemia has been implicated in the formation of drusen, which is characteristic of the early stage of AMD. Several lines of evidence support a role for dyslipidemia in AMD pathogenesis. First, histological evidence has identified that drusen are composed of lipid material [8, 32]. Second, animal experiments have demonstrated that impaired macrophage cholesterol efflux through HDL-mediated reverse cholesterol transport may lead to a pro-angiogenic status in AMD [33, 34]. Third, genetic association studies have identified risk variants in genes involved in lipid metabolism and in the transfer of lipids among lipoproteins, such as hepatic lipase C (LIPC), lipoprotein lipase (LPL), cholesterol ester transferase (CETP), ABC-binding cassettes A1 (ABCA1), and apolipoprotein E (APOE), all of which have been shown to influence the course of AMD pathobiology [35,36,37]. Moreover, previous MR studies have found the dyslipidemia is causal in the transition from intermediate to late AMD [17,18,19]. Additionally, this study emphasizes that serum lipid levels also have a causal effect on early AMD.
This study is mainly based on early AMD and provides additional insights into the causal roles of seven serum lipid biomarkers. A key strength of this study is that we used large-scale datasets with standard protocols to measure lipid biomarkers; this allowed us to systematically evaluate the effects of lipids on AMD risk. Compared with traditional studies, MR findings are less likely to be affected by confounding factors or biases associated with reverse causation. At the same time, our results should be interpreted in light of the limitations of the study. First, as this study was based on participants of European ancestry, the generalizability of our findings to other ethnic groups requires further investigation. Second, in the MR framework, genetically predisposed biomarker changes were assumed to have linear and lifetime effects on AMD risk. Therefore, the potential nonlinear relationships and short-term effects of these biomarkers remain unclear. Third, although biochemical evidence suggests that retina-specific lipid transport is facilitated by proteins regulating systemic lipid metabolism [38], retina-specific lipid concentrations would be more relevant measurements for understanding AMD risk. Further studies are needed to investigate the effects of retina-specific lipid metabolism on AMD risk. Furthermore, blood lipid levels change throughout life. Therefore, long-term follow-up studies are needed to understand the impact of dyslipidemia on AMD risk and progression over time. Finally, analysis of multiple variants does not point to a single mechanism being causal for AMD risk. Nevertheless, our study has important research and clinical implications. Our findings show that plasma HDL-C levels are directly connected to AMD pathogenesis, suggesting that modulating HDL-C metabolism may be a new strategy for delaying or preventing AMD. Although drugs that increase HDL-C levels, such as statins, are used to treat cardiovascular disease [39], their influence on AMD risk has not been reliably duplicated [40]. The inverse relationship between AMD and cardiovascular disease reflects a more complex role of lipid metabolism in the development of AMD. In addition, we did not observe a high genetic correlation between AMD and cardiovascular disease, consistent with previous findings [19]. As a result, lipid-modifying medications must be used with great caution, and additional research into the possible influence of HDL-raising medicines on AMD and its associated phenotypes is required.
Conclusions
This study identifies causal relationships between elevated circulating HDL-C/ApoA levels and increased risk of early AMD, whereas TG specifically decreases the risk of early AMD. Our results also support further investigation into the use of lipid-modifying strategies to prevent or treat early AMD.
Methods
Study overview
This study used a two-sample MR approach to estimate the causal impact of exposures on the outcome using independent GWAS statistics. A total of seven lipid biomarkers, including apolipoprotein A (ApoA), apolipoprotein B (ApoB), total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), lipoprotein A [Lp(a)], and triglycerides (TG), were used as exposures, and early AMD was the outcome. All data used in the current study is publicly available GWAS data, and the relevant ethical approvals can be found in the corresponding studies. Multiple testing was performed by Bonferroni correction, and significance was defined as a P-value < 0.0071 (= 0.05/7).
Genetic instruments for serum lipid biomarkers
GWAS summary datasets for serum lipid biomarkers were obtained from the OpenGWAS database developed by the MRC Integrative Epidemiology Unit (IEU) at the University of Bristol (https://gwas.mrcieu.ac.uk/). Information on each lipid biomarker GWAS is summarized in Table 3. To ensure robust associations between genetic instruments and each lipid biomarker, we selected only genetic variants that reached genome-wide significance (P < 5 × 10−8) for MR analysis. If more than one GWAS dataset was available for a lipid biomarker, we selected the dataset containing the most valid genetic instruments.
GWAS summary statistics for early AMD
GWAS summary data for early AMD was obtained from a recently published genome-wide association meta-analysis which successfully identified multiple novel loci [22]. Winkler and colleagues gathered GWAS data for early AMD from 11 sources, including the International AMD Genomics Consortium (IAMDGC) and UK Biobank (UKBB) [22]. In total, 14,034 cases and 91,214 controls were included in the analysis (number of SNPs = 11,304,110). All included individuals were of European ancestry.
Statistical analysis
To ensure that only uncorrelated variants were included in MR analysis, all variants correlated with the most significant SNPs were excluded (clumping r2 cut-off = 0.001, clumping window = 10,000 kb). Subsequently, all included SNPs were extracted from the GWAS dataset for early AMD, and alleles were harmonized. MR analysis was conducted using the TwoSampleMR R package [41]. The inverse-variance-weighted (IVW) method was the primary analysis method used to assess the causal effect of each lipid biomarker on early AMD.
To assess the potential impact of horizontal pleiotropy, a series of sensitivity analyses were performed, including MR-Egger regression, weighted median method, and weighted mode method [41]. These methods allow for the existence of horizontal pleiotropy but have less statistical power than IVW [41]. Additionally, the Egger intercept was calculated to assess horizontal pleiotropy [42]. MR pleiotropy residual sum and outlier (MR-PRESSO) testing was applied to assess potential bias from outliers and to evaluate overall heterogeneity [43]. Leave-one-out analysis was also applied to assess the influences of particular SNPs [41].
To avoid collinearity, we performed multivariable MR (MVMR)-IVW analysis by selecting groups of exposures. By using genetic instruments associated with any of the included sets of exposures, MVMR analysis can estimate the influence of each exposure condition on the effects of SNPs related to the other exposures.
Availability of data and materials
The dataset(s) supporting the conclusions of this article is(are) included within the article (and its additional file(s)).
Abbreviations
- AMD:
-
Age-related macular degeneration
- GA:
-
Geographic atrophy
- Anti-VEGF:
-
Anti-vascular endothelial growth factor
- MR:
-
Mendelian randomization
- HDL-C:
-
High-density lipoprotein cholesterol
- ApoA:
-
Apolipoprotein A
- ApoB:
-
Apolipoprotein B
- CHOL:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- Lp(a):
-
Lipoprotein A
- TG:
-
Triglycerides
- IAMDGC:
-
International AMD Genomics Consortium
- UKBB:
-
UK Biobank
- IVW:
-
Inverse-variance weighted
- MR-PRESSO:
-
MR pleiotropy residual sum and outlier
- MVMR:
-
Multivariable MR
References
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–38.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–116.
Cruess AF, Zlateva G, Xu X, Soubrane G, Pauleikhoff D, Lotery A, Mones J, Buggage R, Schaefer C, Knight T, et al. Economic burden of bilateral neovascular age-related macular degeneration: multi-country observational study. Pharmacoeconomics. 2008;26(1):57–73.
de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355(14):1474–85.
Curcio CA. Soft drusen in age-related macular degeneration: biology and targeting via the oil spill strategies. Invest Ophthalmol Vis Sci. 2018;59(4):AMD160–81.
Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR. Beckman Initiative for Macular Research Classification C: Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–51.
Group CR, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–908.
Wang L, Clark ME, Crossman DK, Kojima K, Messinger JD, Mobley JA, Curcio CA. Abundant lipid and protein components of drusen. PLoS ONE. 2010;5(4):e10329.
Lekwuwa M, Choudhary M, Lad EM, Malek G: Osteopontin accumulates in basal deposits of human eyes with age-related macular degeneration and may serve as a biomarker of aging. Mod Pathol. 2022;35(2):165–76.
Wang Y, Wang M, Zhang X, Zhang Q, Nie J, Zhang M, Liu X, Ma L: The association between the lipids levels in blood and risk of age-related macular degeneration. Nutrients. 2016;22;8(10):663.
Klein R, Lee KE, Tsai MY, Cruickshanks KJ, Gangnon RE, Klein BEK. Oxidized low-density lipoprotein and the incidence of age-related macular degeneration. Ophthalmology. 2019;126(5):752–8.
Colijn JM, den Hollander AI, Demirkan A, Cougnard-Gregoire A, Verzijden T, Kersten E, Meester-Smoor MA, Merle BMJ, Papageorgiou G, Ahmad S, et al. Increased high-density lipoprotein levels associated with age-related macular degeneration: evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology. 2019;126(3):393–406.
van Leeuwen EM, Emri E, Merle BMJ, Colijn JM, Kersten E, Cougnard-Gregoire A, Dammeier S, Meester-Smoor M, Pool FM, de Jong EK, et al. A new perspective on lipid research in age-related macular degeneration. Prog Retin Eye Res. 2018;67:56–86.
Kelly UL, Grigsby D, Cady MA, Landowski M, Skiba NP, Liu J, Remaley AT, Klingeborn M, Bowes Rickman C. High-density lipoproteins are a potential therapeutic target for age-related macular degeneration. J Biol Chem. 2020;295(39):13601–16.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601.
Pingault JB, O’Reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, Dudbridge F. Using genetic data to strengthen causal inference in observational research. Nat Rev Genet. 2018;19(9):566–80.
Burgess S, Davey Smith G. Mendelian randomization implicates high-density lipoprotein cholesterol-associated mechanisms in etiology of age-related macular degeneration. Ophthalmology. 2017;124(8):1165–74.
Fan Q, Maranville JC, Fritsche L, Sim X, Cheung CMG, Chen LJ, Gorski M, Yamashiro K, Ahn J, Laude A, et al. HDL-cholesterol levels and risk of age-related macular degeneration: a multiethnic genetic study using Mendelian randomization. Int J Epidemiol. 2017;46(6):1891–902.
Han X, Ong JS, Hewitt AW, Gharahkhani P, MacGregor S. The effects of eight serum lipid biomarkers on age-related macular degeneration risk: a Mendelian randomization study. Int J Epidemiol. 2021;50(1):325–36.
Hwang S, Kang SW, Choi J, Son KY, Lim DH, Shin DW, Kim K, Kim SJ. Lipid profile and future risk of exudative age-related macular degeneration development: a nationwide cohort study from South Korea. Sci Rep. 2022;12(1):18777.
Koo E, Neuringer M, SanGiovanni JP. Macular xanthophylls, lipoprotein-related genes, and age-related macular degeneration. Am J Clin Nutr. 2014;100 Suppl 1(1):336S–346S.
Winkler TW, Grassmann F, Brandl C, Kiel C, Gunther F, Strunz T, Weidner L, Zimmermann ME, Korb CA, Poplawski A, et al. Genome-wide association meta-analysis for early age-related macular degeneration highlights novel loci and insights for advanced disease. BMC Med Genomics. 2020;13(1):120.
Calabresi L, Gomaraschi M, Villa B, Omoboni L, Dmitrieff C, Franceschini G. Elevated soluble cellular adhesion molecules in subjects with low HDL-cholesterol. Arterioscler Thromb Vasc Biol. 2002;22(4):656–61.
Parthasarathy S, Barnett J, Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044(2):275–83.
Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41(9):1495–508.
Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32(12):2813–20.
Heinecke JW. The HDL proteome: a marker–and perhaps mediator–of coronary artery disease. J Lipid Res. 2009;50(Suppl):S167–171.
Holzer M, Trieb M, Konya V, Wadsack C, Heinemann A, Marsche G. Aging affects high-density lipoprotein composition and function. Biochim Biophys Acta. 2013;1831(9):1442–8.
Handa JT, Cano M, Wang L, Datta S, Liu T. Lipids, oxidized lipids, oxidation-specific epitopes, and age-related macular degeneration. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(4):430–40.
Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96(6):2758–67.
Vavvas DG, Daniels AB, Kapsala ZG, Goldfarb JW, Ganotakis E, Loewenstein JI, Young LH, Gragoudas ES, Eliott D, Kim IK, et al. Regression of some high-risk features of age-related macular degeneration (AMD) in patients receiving intensive statin treatment. EBioMedicine. 2016;5:198–203.
Curcio CA, Presley JB, Millican CL, Medeiros NE. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005;80(6):761–75.
Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013;17(4):549–61.
Sene A, Apte RS. Eyeballing cholesterol efflux and macrophage function in disease pathogenesis. Trends Endocrinol Metab. 2014;25(3):107–14.
Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107(16):7401–6.
Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci U S A. 2010;107(16):7395–400.
Cheng CY, Yamashiro K, Chen LJ, Ahn J, Huang L, Huang L, Cheung CM, Miyake M, Cackett PD, Yeo IY, et al. New loci and coding variants confer risk for age-related macular degeneration in East Asians. Nat Commun. 2015;6:6063.
Zheng W, Mast N, Saadane A, Pikuleva IA. Pathways of cholesterol homeostasis in mouse retina responsive to dietary and pharmacologic treatments. J Lipid Res. 2015;56(1):81–97.
McTaggart F, Jones P. Effects of statins on high-density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008;22(4):321–38.
Ma L, Wang Y, Du J, Wang M, Zhang R, Fu Y. The association between statin use and risk of age-related macular degeneration. Sci Rep. 2015;5:18280.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R et al: The MR-base platform supports systematic causal inference across the human phenome. Elife 2018, 7.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Acknowledgements
The authors thank all participants and investigators for their contributions to the UK Biobank and International AMD Genomics Consortium and for making summary statistics publicly accessible for this analysis.
Funding
This work was supported by the Zhejiang Provincial Clinical Research Center for Pediatric Disease (2022E50003), Natural Science Foundation of Zhejiang Province (LGF22H120013 and LY21H180004), and the National Natural Science Foundation of China (82201229).
Author information
Authors and Affiliations
Contributions
XFH, QXZ, and QY contributed to the study design. XFH, FFL, YW, LC, CC, and FQR contributed to data collection and analysis. FFL, LJS, QC, LX, and XFH contributed to data interpretation. FFL, YW, and LC wrote the manuscript. XFH, QXZ, and QY revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All data were obtained from open-access resources. GWAS summary datasets of serum lipid biomarkers were obtained from the OpenGWAS database developed by MRC Integrative Epidemiology Unit (IEU) at the University of Bristol (https://gwas.mrcieu.ac.uk/). Winkler and colleagues gathered GWAS data for early AMD from 11 sources, including the International AMD Genomics Consortium (IAMDGC) and UK Biobank (UKBB).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, FF., Wang, Y., Chen, L. et al. Causal effects of serum lipid biomarkers on early age-related macular degeneration using Mendelian randomization. Genes Nutr 18, 11 (2023). https://doi.org/10.1186/s12263-023-00730-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12263-023-00730-5