Abstract
Background
Chronic recurrent multifocal osteomyelitis (CRMO) is a rare autoinflammatory bone disease requiring immunosuppressive treatment in half of patients. Monoclonal tumor necrosis factor inhibitors (TNFi) are often used as effective second-line off-label therapies. However, paradoxical psoriasis can occur in a subset of patients exposed to monoclonal TNFi and can prompt conversion to alternate therapy if severe.
Objective
The aim of this study was to determine the efficacy and safety of golimumab, a fully humanized TNFi, in children with CRMO, including those who develop paradoxical psoriasis after exposure to other monoclonal TNFi.
Methods
A retrospective chart review was conducted of patients with CRMO who received golimumab in a single center between 01 June, 2018 and 31 December, 2020. Patients who were diagnosed before 21 years of age and followed up for CRMO at least once after receiving ≥ 3 months of golimumab were included. Extracted data included patient demographics, whole-body MRI lesion counts, clinically relevant data, laboratory results, patient-reported outcomes, and psoriasis burden. Linear mixed models with log-transformed outcomes were used to assess changes in the outcomes over time. The random effect is included in the model to account for the within-subject correlation of repeated measures. p-values and 95% confidence intervals were reported.
Results
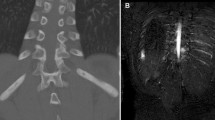
Eighteen patients were included. Patients were observed for a median of 9.95 months [interquartile range 3.84–15.64]. The median age at the initiation of golimumab was 10.95 years [9.86–13.77] and the median duration of disease between the disease onset and the initiation of golimumab was 2.60 years [1.66–3.62]. Ten patients received golimumab via intravenous route and eight patients received golimumab via subcutaneous route. The median dose was 1.64 mg/kg/month [1.46, 2]. Fourteen patients were previously treated with disease-modifying antirheumatic drugs and 17 with other TNFi. Patients treated with golimumab showed significant improvement in median physician global assessment for CRMO from 2.00 [1.00–3.00] to 0.00 [0.00–0.25] by the fourth visit (p < 0.001), with median erythrocyte sedimentation rate (ESR) decreasing significantly from 12.00 [6.75–23.75] to 5.00 [3.00–10.00] by the fourth visit (p < 0.05). The median number of lesions on MRI decreased significantly from 3.50 [2.00–5.50] to 0.50 [0.00–4.25] lesions per patient (p < 0.01). Nine out of 12 patients who had previous paradoxical psoriasis associated with adalimumab or infliximab had persistent active psoriasis at study baseline. For patients with psoriasis at study baseline, the prevalence of psoriasis had decreased from 100% to approximately 50–57% at the following visits. Of the 18 patients initiated on golimumab in this study, there was only one new case of mild psoriasis in a patient with previously resolved infliximab-associated paradoxical psoriasis. No serious infections or adverse events were noted during the study. Two patients in the study showed clinical improvement with concomitant golimumab and ustekinumab with no reported adverse side effects or increased effects in these patients over a 16-month interval, showing that this combination can be safe and effective for children with CRMO.
Conclusion
In our experience, golimumab has been shown to be a safe and effective therapy for CRMO and demonstrated improvement in paradoxical psoriasis in many patients. Longer follow-up periods would be helpful to develop longer term outcomes data for patients with CRMO and overall paradoxical psoriasis risk.


Similar content being viewed by others
References
Hedrich CM, Morbach H, Reiser C, Girschick HJ. New insights into adult and paediatric chronic non-bacterial osteomyelitis CNO. Curr Rheumatol Rep. 2020;22(9):52. https://doi.org/10.1007/s11926-020-00928-1.
Voit AM, Arnoldi AP, Douis H, Bleisteiner F, Jansson MK, Reiser MF, et al. Whole-body Magnetic Resonance Imaging in Chronic Recurrent Multifocal Osteomyelitis: Clinical Longterm Assessment May Underestimate Activity. J Rheumatol. 2015;42:1455–62.
Wipff J, Costantino F, Lemelle I, Pajot C, Duquesne A, Lorrot M, et al. A large national cohort of french patients with chronic recurrent multifocal osteitis. Arthritis Rheumatol. 2015;67:1128–37.
Borzutzky A, Stern S, Reiff A, Zurakowski D, Steinberg EA, Dedeoglu F, et al. Pediatric chronic nonbacterial osteomyelitis. Pediatrics. 2012;130:e1190–7.
Aden S, Wong S, Yang C, Bui T, Higa T, Scheck J, et al. Increasing cases of chronic nonbacterial osteomyelitis in children: a series of 215 cases from a single tertiary referral center. J Rheumatol. 2022;49:929–34.
Jansson AF, Grote V. Nonbacterial osteitis in children: data of a German Incidence Surveillance Study. Acta Paediatr. 2011;100:1150–7.
Girschick H, Finetti M, Orlando F, Schalm S, Insalaco A, Ganser G, et al. The multifaceted presentation of chronic recurrent multifocal osteomyelitis: a series of 486 cases from the Eurofever international registry. Rheumatology. 2018;57:1504–1504.
Beck C, Morbach H, Beer M, Stenzel M, Tappe D, Gattenlöhner S, et al. Chronic nonbacterial osteomyelitis in childhood: prospective follow-up during the first year of anti-inflammatory treatment. Arthritis Res Ther. 2010;12:R74.
Zhao Y, Wu EY, Oliver MS, Cooper AM, Basiaga ML, Vora SS, et al. Consensus treatment plans for chronic nonbacterial osteomyelitis refractory to nonsteroidal antiinflammatory drugs and/or with active spinal lesions. Arthritis Care Res (Hoboken). 2018;70:1228–37.
Zhao Y, Chauvin NA, Jaramillo D, Burnham JM. Aggressive therapy reduces disease activity without skeletal damage progression in chronic nonbacterial osteomyelitis. J Rheumatol. 2015;42:1245–51.
Bhat CS, Roderick M, Sen ES, Finn A, Ramanan AV. Efficacy of pamidronate in children with chronic non-bacterial osteitis using whole body MRI as a marker of disease activity. Pediatr Rheumatol. 2019;17:35.
Gaal A, Basiaga ML, Zhao Y, Egbert M. Pediatric chronic nonbacterial osteomyelitis of the mandible: Seattle Children’s hospital 22-patient experience. Pediatr Rheumatol. 2020;18:4.
Campbell JA, Kodama SS, Gupta D, Zhao Y. Case series of psoriasis associated with tumor necrosis factor-α inhibitors in children with chronic recurrent multifocal osteomyelitis. JAAD Case Rep. 2018;4:767–71.
Buckley LH, Xiao R, Perman MJ, Grossman AB, Weiss PF. Psoriasis associated with tumor necrosis factor inhibitors in children with inflammatory diseases. Arthritis Care Res (Hoboken). 2021;73:215–20.
Brown G, Wang E, Leon A, Huynh M, Wehner M, Matro R, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334–41.
Zhao Y, Sato TS, Nielsen SM, Beer M, Huang M, Iyer RS, et al. Development of a scoring tool for chronic nonbacterial osteomyelitis magnetic resonance imaging and evaluation of its interrater reliability. J Rheumatol. 2020;47:739–47.
Batu ED, Ergen FB, Gulhan B, Topaloglu R, Aydingoz U, Ozen S. Etanercept treatment in five cases of refractory chronic recurrent multifocal osteomyelitis (CRMO). Joint Bone Spine. 2015;82:471–3.
Schnabel A, Nashawi M, Anderson C, Felsenstein S, Lamoudi M, Poole-Cowley J, et al. TNF-inhibitors or bisphosphonates in chronic nonbacterial osteomyelitis?—results of an international retrospective multicenter study. Clin Immunol. 2022;238: 109018.
Rosenwasser N, Lee D, Sidbury R, Zhao Y. Paradoxical psoriasis in children receiving Anti-TNFα treatment for inflammatory/autoimmune disease. Pediatr Drugs. 2021;23:131–41.
Kodama S, Gupta D, Sullivan E, Rosenwasser N, Zhao Y. Paradoxical psoriasis after exposure to tumour necrosis factor inhibitors in children: a retrospective cohort study. Br J Dermatol. 2022;186:1043–5.
Baggett K, Brandon TG, Xiao R, Valenzuela Z, Buckley LH, Weiss PF. Incidence rates of psoriasis in children with inflammatory bowel disease and juvenile arthritis treated with tumor necrosis factor inhibitors and disease-modifying antirheumatic drugs. J Rheumatol. 2022;49:935–41.
Groth D, Perez M, Treat JR, Castelo-Soccio L, Nativ S, Weiss PF, et al. Tumor necrosis factor-α inhibitor-induced psoriasis in juvenile idiopathic arthritis patients. Pediatr Dermatol. 2019;36:613–7.
Olbjørn C, Rove JB, Jahnsen J. Combination of biological agents in moderate to severe pediatric inflammatory bowel disease: a case series and review of the literature. Pediatr Drugs. 2020;22:409–16.
Acknowledgements
We thank all patients and their families for participating in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no dedicated funding for this research. CRMO Warriors Guild has provided support for a research assistant and statistician.
Conflict of interest:
The authors declare that they have no conflict of interest.
Availability of data and materials:
Data are available to the public when a reasonable request is made.
Ethics Approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Seattle Children’s Hospital approved this study.
Consent to Participate
Informed consent and assent were obtained from all individual participants and legal guardians included in the study.
Consent for Publication
Patients signed informed consent regarding publishing their data and photographs.
Code availability:
Not applicable.
Author Contributions
Conceptualization: CY, YZ. Data collection: CY, NR, MDB, DG, HAB, RS, RSI, YZ. Data analysis: XW, ZX. Writing: CY, NR, YZ. All authors read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Rosenwasser, N., Wang, X. et al. Golimumab in Children with Chronic Recurrent Multifocal Osteomyelitis: A Case Series and Review of the Literature. Pediatr Drugs 25, 603–611 (2023). https://doi.org/10.1007/s40272-023-00581-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00581-y