Abstract
Immediate lymphatic reconstruction (ILR) at the time of axillary lymph node dissection (ALND) has become increasingly utilized for the prevention of breast cancer related lymphedema. Preoperative indocyanine green (ICG) lymphography is routinely performed prior to an ILR procedure to characterize baseline lymphatic anatomy of the upper extremity. While most patients have linear lymphatic channels visualized on ICG, representing a non-diseased state, some patients demonstrate non-linear patterns. This study aims to determine potential inciting factors that help explain why some patients have non-linear patterns, and what these patterns represent regarding the relative risk of developing postoperative breast cancer related lymphedema in this population. A retrospective review was conducted to identify breast cancer patients who underwent successful ILR with preoperative ICG at our institution from November 2017—June 2022. Among the 248 patients who were identified, 13 (5%) had preoperative non-linear lymphatic anatomy. A history of trauma or surgery of the affected limb and an increasing number of sentinel lymph nodes removed prior to ALND appeared to be risk factors for non-linear lymphatic anatomy. Furthermore, non-linear anatomy in the limb of interest was associated with an increased risk of postoperative lymphedema development. Overall, non-linear lymphatic anatomy on pre-operative ICG lymphography appears to be a risk factor for developing ipsilateral breast cancer-related lymphedema. Guided by the study’s findings, when breast cancer patients present with baseline non-linear lymphatic anatomy, our institution has implemented a protocol of prophylactically prescribing compression sleeves immediately following ALND.
Similar content being viewed by others
Introduction
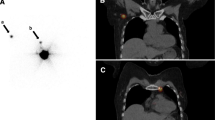
Immediate lymphatic reconstruction (ILR) has become increasingly recognized and adopted by lymphatic microsurgeons for the prevention of breast cancer related lymphedema. ILR involves anastomosing divided arm lymphatic channels to an adjacent vein at the time of axillary lymph node dissection (ALND), thereby restoring a pathway for lymphatic drainage [1]. As part of the preoperative evaluation prior to ILR, indocyanine green (ICG) lymphography of the upper extremities is utilized for visualization and anatomical mapping of a patient’s superficial lymphatic system [2,3,4,5,6,7]. Mapping of the lymphatic anatomy is done as part of our standard of care in order to (1) document an individual’s baseline anatomy and (2) serve as a potential guide for ICG-guided manual lymphatic drainage (MLD) or other therapies if the patient were to develop lymphedema. Multiple morphologies of lymphatic drainage on ICG lymphography have been described in the literature, including both linear and non-linear lymphatic channels (Fig. 1) [8] Linear channels are characterized by longitudinal fluorescent lines and typically reflect healthy lymphatic collectors [8,9,10]. In contrast, non-linear morphologies represent pathologic lymphatic obstruction and are associated with more severe stages of lymphedema [9, 11,12,13]. Interestingly, a small subset of breast cancer patients without prior history of lymphatic disease have been noted to have non-linear lymphatic anatomy at a baseline state, prior to undergoing ALND [3, 14]. The potential implications of these findings on lymphedema risk after ALND have not been previously studied.
Linear and Non-Linear Lymphatic Anatomy Demonstrated Immediately Prior to Axillary Lymph Node Dissection with Pre-operative Indocyanine Green (ICG) Lymphography. A Linear lymphatic anatomy on ICG lymphography, B Non-linear lymphatic anatomy on ICG lymphography. Note: Figures were obtained from patients treated at the sponsoring institution. Permissions to utilize their study contents for the purpose of research and publication were obtained. Animated images were developed for the use of this study using Canva, an online design software
Within the subset of non-linear morphology, also referred to as dermal backflow (DBF), the lymphatic drainage can be further differentiated as splash, stardust, or diffuse patterns (Fig. 2) [8]. Prior studies have suggested that with increasing severity of lymphedema, DBF patterns will follow a sequential evolution from splash to stardust to diffuse patterning [15, 16]. Prior studies on non-linear lymphatic anatomy have been particularly useful for developing methods for the early detection of lymphedema as well as for staging and grading the disease severity [15, 16]. However, investigations to date have not yet examined the implications of non-linear morphologies present in patients without lymphedema. Therefore, the effects of baseline non-linear morphologies on the risk of lymphedema development after ALND is unknown.
Demonstration of the Various Types of Upper Extremity Lymphatic Drainage as it would be Visualized with Indocyanine Green (ICG) Lymphography. A Diffuse patterning, B Stardust patterning, C Splash patterning, D Linear lymphatic patterning. Note: Figures were created for the use of this publication in Adobe Photoshop
Recent studies have demonstrated that ILR can significantly decrease the risk of breast cancer related lymphedema in patients from a 33% incidence to a 9% incidence [1, 3, 16, 17]. However, the incidence of breast cancer related lymphedema among patients with preoperative non-linear anatomy is not well understood, including whether or not they receive the same benefit from ILR as breast cancer patients with linear anatomy. Therefore, this study aims to determine the incidence of breast cancer related lymphedema in patients with preoperative non-linear lymphatic anatomy in order to better understand the clinical significance and implications of these morphologies on patients’ risk of lymphedema.
Methods
Retrospective Review
A retrospective study was conducted at the Boston Lymphatic Center, Beth Israel Deaconess Medical Center. Institutional review board approval was obtained for this study (Protocol #2022P000450). A review of a prospectively maintained REDCap Quality Improvement Database [18] and electronic medical records was performed. Patients who received ICG lymphography prior to ALND and attempted ILR from November 2017 to June 2022 were identified. Patient demographics, prior medical and surgical history, operative records, and postoperative outcomes were extracted. A history of prior trauma to the limb of interest was recorded. Prior trauma was defined as having any injury, procedure, or surgical intervention on the upper limb undergoing the ICG lymphography, from the axillary region to the hand.
ICG Lymphography
Prior to ALND, under sterile conditions, 0.1 cc of stock (2.5 mg/cc) ICG solution (Akorn Inc., Lake Forest, IL) mixed with 5% of albumin was injected intradermally. The locations of these injections were based on the lymphosome concept, and represent all the previously identified zones of the hand, forearm, and lateral upper arm pathway [2, 19]. By injecting each lymphosome, the major lymphatic drainage pathways of the hand and forearm are represented [19]. A near-infrared (NIR) imaging device, the PDE-Neo II (Hamamatsu Photonics KK, Hamamatsu, Japan) was used to visualize the superficial lymphatic channels of the extremity. All ICG lymphographies were performed by one of two members of the lymphatic surgery team (ET or DS) and interpreted by the same lymphatic surgeon (DS). The readings are recorded via a REDCap form for quantitative analysis of the patient population.
All ALNDs were performed by the same institutional general surgery team. After conclusion of ALND, ILR was attempted by the same lymphatic surgeon (DS). Patients in which ILR was intraoperatively aborted were excluded from the analysis. Following ILR, all patients entered our postoperative surveillance program for monitoring of lymphedema development as described in prior investigations [20]. A diagnosis of lymphedema was defined as the presence of symptoms consistent with lymphedema, such as pain, swelling, restricted range of motion, trouble fitting into clothing, feelings of heaviness or tightness, and one of the following objective findings: (1) relative limb volume change greater than 10% or (2) Lymphedema Index (L-Dex) greater than 10 from baseline. All measurements and physical exams were performed by a certified lymphedema therapist.
Statistical Analysis
The data were separated into two cohorts based on whether patients had linear or non-linear findings on upper extremity ICG lymphography. To assess for differences between categorical variables, a Fisher exact test was performed. To assess for differences between continuous variables, Wilcoxon rank sum test was used. Multivariate logistic regression was utilized to assess the association between a history of trauma and the number of sentinel lymph nodes removed, with the odds of having a non-linear ICG lymphography finding. All analyses were conducted using R statistical programming language (version 4.2.1, R Core Team 2021, Vienna, Austria) and SAS Studio (Version 3.81, SAS Institute Inc 2013, Cary, NC, USA) [21]. Statistical significance was set at α = 0.05.
Results
During the study period, a total of 275 patients had preoperative upper extremity ICG lymphography and ALND with an attempted ILR over the study period at our institution. Among these patients, 27 (10%) had an aborted ILR procedure and were excluded from the study. Towards the beginning of the study period, aborting the procedure was commonly due to inability to create the lymphovenous anastomosis under minimal tension. Since encorporating our new technique of utilizing a lower extremity vein graft to the anastamosis, aborted ILR procedures have been greatly decreased [22]. Notably, none of these patients had an aborted ILR due to lymphatic anatomy. Therefore, a total of 248 patients were included. The median age and body mass index (BMI) of the population was 57 years and 27 m/kg2 respectively. The vast majority of patients were female (97%). On preoperative ICG lymphography, 13 patients (5%) had preoperative non-linear lymphatic anatomy within the limb of interest. Demographics were similar between the linear and non-linear cohorts (Table 1).
When comparing the linear and non-linear cohorts, a greater proportion of the non-linear cohort had a history of trauma, injury, or invasive procedures (46%) to the upper extremity compared to the linear cohort (11%) (p = 0.004). A comprehensive list of upper extremity procedures, injuries, and traumas for each cohort is displayed in Fig. 3.
Regarding oncologic evaluation, the two cohorts had comparable rates of receiving sentinel lymph node biopsies (54% of the linear cohort compared to 46% of the non-linear cohort). Notably, the median number of sentinel lymph nodes removed was higher in the non-linear cohort (6) compared to the linear cohort (3) (Table 2). Logistic regression modeling demonstrated that the total number of sentinel lymph nodes removed was associated with 1.2 odds of having a non-linear anatomy (p = 0.02). Additionally, having trauma to the limb of interest was associated with 6.0 increased odds of having non-linear lymphatic anatomy (p = 0.020). The number of positive nodes removed during the sentinel lymph node biopsy, the number of nodes removed during the ALND, and the number of positive nodes removed during ALND were similar across the groups.
Non-Linear Subgroup Analysis
The 13 patients with non-linear lymphatic anatomy were assessed for incidence of postoperative lymphedema development. All patients were female (100%) with an average age of 57.2 years (SD 10.8), and an average body mass index (BMI) of 31.9 kg/m2 (SD 6.1). All participants received adjuvant radiation (100%), 8 patients (67%) received neoadjuvant chemotherapy, and 8 patients (67%) received adjuvant chemotherapy. Regarding the postoperative incidence of lymphedema, one patient had neoadjuvant taxol-induced lymphedema prior to ILR and was therefore excluded from the investigation of lymphedema incidence. The remaining 12 patients reported no symptoms of lymphedema prior to ILR and were therefore included.
Of the 12 included patients, the total number of non-linear patients meeting clinical criteria for lymphedema was 5 (42%) and 4 (33%) did not. Notably, the remaining 3 (25%) patients did not meet all clinical criteria for lymphedema diagnosis, but showed signs of lymphedema with either increase in objective values (L-Dex > 10%, a relative volume change > 10% in the limb of interest) or had symptoms consistent with lymphedema. These patients were classified as having “sub-clinical lymphedema.” The average follow-up time was 10.8 months (SD 7.3). The average time from ILR to lymphedema diagnosis was 6.7 months (SD 3.0).
Discussion
In this study, we investigated the implications of preoperative non-linear upper extremity lymphatic anatomy on the incidence of breast cancer-related lymphedema. Our findings demonstrate that patients with non-linear anatomy (1) had a higher rate of prior surgery or trauma to the limb, (2) a greater number of lymph nodes removed during preoperative sentinel lymph node biopsy, and (3) an increased incidence of lymphedema following ALND and ILR.
This study suggests a possible association between prior trauma or injury to the superficial lymphatic channels correlating with a higher risk of lymphedema following oncologic breast interventions. Understanding the association between the surgical treatment for breast cancer and the development of lymphedema is critical, as one in five patients develop upper extremity lymphedema after lymph node extraction and mastectomy [1, 23]. Our Lymphatic Center, a certified Center of Excellence, invests significant focus on researching surgical and non-invasive interventions associated with reduced rates of lymphedema, thereby allowing for robust investigations into this patient population [24]. Our center has previously observed that of the breast cancer patients who had received a successful ILR procedure after ALND by the same surgeon, 9% of the cohort went on to develop post-operative lymphedema after 11 months of followup [17]. Notably, in this study’s assessment of patients specifically with non-linear anatomy, 42% of patients developed a clinical lymphedema diagnosis with an additional 25% showing signs of lymphedema. Additionally, these patients had a shorter follow-up compared to those patients in the previous study (10.5 months compared to 17 months) [17]. As lymphedema can develop in a delayed fashion, having a larger incidence in breast cancer related lymphedema with a significantly shorter follow-up is likely to underestimate the incidence of breast cancer related lymphedema in this population. This critical difference in breast cancer related lymphedema incidence suggests that preoperative non-linear anatomy may be an independent risk factor for lymphedema.
There are known risk factors for breast cancer-related lymphedema, including (1) axillary lymph node dissection, (2) regional lymph node radiation (RLNR), and (3) high BMI [23, 25,26,27]. Other debated risk factors include (1) taxane-based chemotherapy, (2) increasing age, and (3) modified radical mastectomy [28,29,30]. Our findings suggest that prior trauma or invasive procedures to the ipsilateral arm or axillary region is an important additional risk factor for the development of breast cancer-related lymphedema. Prior trauma could incite lymphatic dysfunction that can be further perturbed with direct mechanical force or manipulation during breast cancer procedures. Further research is required to investigate the coorlation of these traumas to the lymphosomes with resultant non-linear lymaphatic anatomy [19]. These results seem to suggest that the presence of non-linear lymphatic anatomy, regardless of the cause or prior trauma history, may be a risk factor for breast cancer-related lymphedema.
These conclusions align with existing literature stating that non-linear patterns represent dysfunctional lymphatic flow [15, 16]. Furthermore, Buchan et. al found that even in patients with pre-operative linear lymphatic anatomy, lymphovenous bypass during ALND may result in changes to the lymphatic anatomy in select patients’ post-operative ICG readings [31]. The authors of this article postulate that these patients may have had risk factors such as minor trauma to the limb that could have lowered the threshould to incite post operative lymphatic flow changes. This potentially supports the theory this paper presents as trauma to the limb of interest could be a risk factor for lymphatic flow disruption.
Globally, patient accessibility to a surgeon trained to perform lymphatic surgery is limited. While lymphedema has gained increasing recognition as a major issue faced by one in five breast cancer survivors, there remains a critical deficit of trained microsurgeons able to perform risk-reducing ILR to meet the needs of these patients [32,33,34]. Therefore, research focusing on non-invasive, preventative approaches for lymphedema is imperative to target breast cancer patients without access to a lymphatic surgeon or insurance coverage for ILR. Furthermore, continuing collaboration within the fields of plastic surgery, breast surgery, oncology, radiology, and physical therapy is necessary to decrease the risk of lymphedema in this patient population.
Our results suggest that prophylactic interventions may benefit patients with preoperative non-linear lymphatic anatomy, as they appear to be at high risk for lymphedema development. At our Lymphatic Center, patients presenting with non-linear lymphatic anatomy begin prophylactic compression therapy of the affected limb immediately after ALND and ILR. This protocol aims to help target and prevent lymphedema for high-risk patients. The use of compression garments immediately after ALND has been found to significantly decrease the incidence of lymphedema in breast cancer patients, though it is well documented that compression garments have detrimental effects on patient quality of life and compliance levels can vary [35,36,37]. In institutions where ICG lymphangiography or ILR may not be available, our findings suggest that patients with previous trauma to the limb or those with a large number of nodes removed in a prior SNLB may particularly benefit from early use of compression garments prophylactically. Additionally, diligent follow-up and monitoring can aid in early detection of lymphedema, allowing for earlier intervention.
Limitations
This study must be interpreted in the context of several limitations. As non-linear lymphatic anatomy on pre-operative ICG lymphography is an uncommon phenomenon, the statistical analysis is underpowered. Nevertheless, our results quantitively demonstrate an association between lymphedema development and non-linear lymphatic anatomy. Our team anticipates a steady growth in the number of patients with non-linear findings, as there is an increasing volume of patients being offered ILR at our institution and greater opportunities for detection through longer follow-up.
Conclusion
Non-linear lymphatic anatomy on preoperative ICG lymphography appears to be a risk factor for the development of breast cancer related lymphedema. Patients with non-linear anatomy tended to have a history of trauma, procedures, or injury to the ipsilateral arm. Additionally, these patients were more likely to have a larger burden of sentinel lymph nodes excised prior to ALND. Guided by these findings, we have begun to prophylactically prescribe compression garments immediately postoperatively to patients with non-linear lymphatic anatomy on preoperative ICG lymphography in an effort to target and prevent lymphedema development in this high-risk population.
Availability of Data and Materials
N/A.
References
Johnson AR, Fleishman A, Granoff MD, et al. Evaluating the Impact of Immediate Lymphatic Reconstruction for the Surgical Prevention of Lymphedema. Plast Reconstr Surg. 2021;147(3):373e–81e. https://doi.org/10.1097/PRS.0000000000007636.
Johnson AR, Granoff MD, Suami H, Lee BT, Singhal D. Real-Time Visualization of the Mascagni-Sappey Pathway Utilizing ICG Lymphography. Cancers. 2020;12(5):1195. https://doi.org/10.3390/cancers12051195.
Granoff MD, Pardo JA, Johnson AR, et al. Superficial and Functional Lymphatic Anatomy of the Upper Extremity. Plast Reconstr Surg. 2022;150(4):900–7. https://doi.org/10.1097/PRS.0000000000009555.
Hara H, Mihara M. Indocyanine Green Lymphographic and Lymphoscintigraphic Findings in Genital Lymphedema-Genital Pathway Score. Lymphat Res Biol. 2017;15(4):356–9. https://doi.org/10.1089/lrb.2017.0025.
Tashiro K, Yamashita S, Koshima I, Miyamoto S. Visualization of Accessory Lymphatic Pathways in Secondary Upper Extremity Lymphedema Using Indocyanine Green Lymphography. Ann Plast Surg. 2017;79(4):393–6. https://doi.org/10.1097/SAP.0000000000001120.
Hara H, Mihara M. Multilymphosome Injection Indocyanine Green Lymphography Can Detect More Lymphatic Vessels Than Lymphoscintigraphy in Lymphedematous Limbs. J Plast Reconstr Aesthetic Surg JPRAS. 2020;73(6):1025–30. https://doi.org/10.1016/j.bjps.2020.01.021.
Friedman R, Bustos VP, Pardo J, et al. Superficial and Functional Imaging of the Tricipital Lymphatic Pathway: A Modern Reintroduction. Breast Cancer Res Treat. Published online November 3, 2022. https://doi.org/10.1007/s10549-022-06777-z.
Yamamoto T, Yamamoto N, Doi K, et al. INdocyanine Green-enhanced Lymphography for Upper Extremity Lymphedema: a Novel Severity Staging System Using Dermal Backflow Patterns. Plast Reconstr Surg. 2011;128(4):941–7. https://doi.org/10.1097/PRS.0b013e3182268cd9.
Narushima M, Yamamoto T, Ogata F, Yoshimatsu H, Mihara M, Koshima I. Indocyanine Green Lymphography Findings in Limb Lymphedema. J Reconstr Microsurg. 2016;32(1):72–9. https://doi.org/10.1055/s-0035-1564608.
Heydon-White A, Suami H, Boyages J, Koelmeyer L, Peebles KC. Assessing Breast Lymphoedema Following Breast Cancer Treatment Using Indocyanine Green Lymphography. Breast Cancer Res Treat. 2020;181(3):635–44. https://doi.org/10.1007/s10549-020-05661-y.
Mihara M, Seki Y, Hara H, et al. Predictive Lymphatic Mapping: a Method for Mapping Lymphatic Channels in Patients With Advanced Unilateral Lymphedema Using Indocyanine Green Lymphography. Ann Plast Surg. 2014;72(6):706–10. https://doi.org/10.1097/SAP.0b013e31826a18b1.
Suami H, Koelmeyer L, Mackie H, Boyages J. Patterns of Lymphatic Drainage After Axillary Node Dissection Impact Arm Lymphoedema Severity: A Review of Animal and Clinical Imaging Studies. Surg Oncol. 2018;27(4):743–50. https://doi.org/10.1016/j.suronc.2018.10.006.
Shinaoka A, Koshimune S, Yamada K, et al. Accelerated Lymph Flow in Early-Stage Secondary Lymphedema Detected by Indocyanine Green Fluorescence Lymphography. J Reconstr Microsurg. 2017;33(8):596–602. https://doi.org/10.1055/s-0037-1603740.
Granoff MD, Pardo J, Shillue K, et al. Variable Anatomy of the Lateral Upper Arm Lymphatic Channel: A Potential Anatomic Risk Factor for the Development of Breast Cancer Related Lymphedema. Plast Reconstr Surg. Published online January 24, 2023. https://doi.org/10.1097/PRS.0000000000010245.
Early Diagnosis and Risk Factors for Lymphedema Following Lymph Node Dissection for Gynecologic Cancer - PubMed. https://pubmed.ncbi.nlm.nih.gov/23357989/. Accessed 12 Dec 2022.
The Earliest Finding of Indocyanine Green Lymphography in As... : Plastic and Reconstructive Surgery. https://journals.lww.com/plasreconsurg/Abstract/2011/10000/The_Earliest_Finding_of_Indocyanine_Green.25.aspx. Accessed 12 Dec 2022.
Granoff MD, Fleishman A, Shillue K, et al. A four-year institutional experience of immediate lymphatic reconstruction. Plast Reconstr Surg. 2023. https://doi.org/10.1097/PRS.0000000000010381.
The Boston Lymphatic Center’s Early Experience With Lymph Node Transplantation to the Upper Extremity. https://parjournal.net/article/view/5233. Accessed 6 Dec 2022.
Suami H, Taylor GI, Pan WR. The lymphatic territories of the upper limb: anatomical study and clinical implications. Plast Reconstr Surg. 2007;119(6):1813–22. https://doi.org/10.1097/01.prs.0000246516.64780.61.
Developing a Lymphatic Surgery Program: A First-Year Review : Plastic and Reconstructive Surgery. https://journals.lww.com/plasreconsurg/Abstract/2019/12000/Developing_a_Lymphatic_Surgery_Program__A.6.aspx. Accessed 6 Dec 2022.
R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 5 Sept 2022.
Friedman R, Bustos VP, Postian T, et al. Utilizing a lower extremity vein graft for immediate lymphatic reconstruction. J Plast Reconstr Aesthetic Surg JPRAS. 2022;75(8):2831–70. https://doi.org/10.1016/j.bjps.2022.06.076.
Johnson AR, Kimball S, Epstein S, et al. Lymphedema Incidence After Axillary Lymph Node Dissection: Quantifying the Impact of Radiation and the Lymphatic Microsurgical Preventive Healing Approach. Ann Plast Surg. 2019;82(4S Suppl 3):S234–41. https://doi.org/10.1097/SAP.0000000000001864.
Chang D, Dayan J, Fried P, et al. Establishing Standards for Centers of Excellence for the Diagnosis and Treatment of Lymphatic Disease. Lymphat Res Biol. 2021;19(1):4–10. https://doi.org/10.1089/lrb.2020.0022.
Wu R, Huang X, Dong X, Zhang H, Zhuang L. Obese patients have higher risk of breast cancer-related lymphedema than overweight patients after breast cancer: a meta-analysis. Ann Transl Med. 2019;7(8):172. https://doi.org/10.21037/atm.2019.03.44.
Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat. 2011;130(3):981–91. https://doi.org/10.1007/s10549-011-1667-z.
Allam O, Park KE, Chandler L, et al. The impact of radiation on lymphedema: a review of the literature. Gland Surg. 2020;9(2):596–602. https://doi.org/10.21037/gs.2020.03.20.
Cariati M, Bains SK, Grootendorst MR, et al. Adjuvant taxanes and the development of breast cancer-related arm lymphoedema. Br J Surg. 2015;102(9):1071–8. https://doi.org/10.1002/bjs.9846.
Guliyeva G, Huayllani MT, Boczar D, Avila FR, Lu X, Forte AJ. Age As a Risk Factor for Breast Cancer-related Lymphedema: A Systematic Review. J Cancer Surviv Res Pract. Published online January 24, 2021. https://doi.org/10.1007/s11764-021-00994-z.
Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GKD, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–72. https://doi.org/10.1245/s10434-009-0452-2.
Buchan G, Cakmakoglu C, Schwarz GS. ICG lymphographic findings following immediate lymphatic reconstruction in breast cancer patients. J Plast Reconstr Aesthetic Surg JPRAS. 2022;75(7):2164–71. https://doi.org/10.1016/j.bjps.2022.02.020.
DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–15. https://doi.org/10.1016/S1470-2045(13)70076-7.
Schulze H, Nacke M, Gutenbrunner C, Hadamitzky C. Worldwide assessment of healthcare personnel dealing with lymphoedema. Health Econ Rev. 2018;8:10. https://doi.org/10.1186/s13561-018-0194-6.
CDCBreastCancer. Basic Information About Breast Cancer. Centers for Disease Control and Prevention. Published October 31, 2022. https://www.cdc.gov/cancer/breast/basic_info/index.htm. Accessed 20 Dec 2022.
Nadal Castells MJ, Ramirez Mirabal E, CuarteroArchs J, et al. Effectiveness of Lymphedema Prevention Programs With Compression Garment After Lymphatic Node Dissection in Breast Cancer: A Randomized Controlled Clinical Trial. Front Rehabil Sci. 2021;2:727256. https://doi.org/10.3389/fresc.2021.727256.
Schaverien MV, Moeller JA, Cleveland SD. Nonoperative Treatment of Lymphedema. Semin Plast Surg. 2018;32(1):17–21. https://doi.org/10.1055/s-0038-1635119.
Paramanandam VS, Dylke E, Clark GM, et al. Prophylactic Use of Compression Sleeves Reduces the Incidence of Arm Swelling in Women at High Risk of Breast Cancer-Related Lymphedema: A Randomized Controlled Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2022;40(18):2004–12. https://doi.org/10.1200/JCO.21.02567.
Funding
Research reported in this publication was supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL157991 (D.S.) and the NIH Common Fund under award number U54HL165440 (D.S.). Rosie Friedman is supported by the 2023 JOBST Lymphatic Research Grant awarded by the Boston Lymphatic Symposium, Inc.
Author information
Authors and Affiliations
Contributions
Made substantial contributions to completion or design of the work: All authors. Performed data acquisition, analysis, interpretation of data for the work: All authors. Helped with drafting or revision of manuscript for important intellectual content: All authors. Provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
No ethical approval was required for this.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kinney, J.R., Friedman, R., Kim, E. et al. Non-Linear Lymphatic Anatomy in Breast Cancer Patients Prior to Axillary Lymph Node Dissection: A Risk Factor For Lymphedema Development. J Mammary Gland Biol Neoplasia 28, 20 (2023). https://doi.org/10.1007/s10911-023-09545-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10911-023-09545-x