Abstract
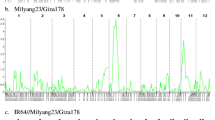
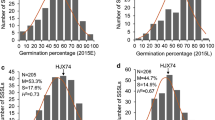
Global warming threatens human life on many aspects, including heat stress on crop production. Breeding heat-tolerant varieties is a fundamental way to meet the challenge. To study the genetic basis of seedling tolerance to heat stress in rice (Oryza sativa L.), we performed QTL mapping based on a large F2 population consisting of 4450 individuals derived from a cross between a japonica rice variety Huaidao 5 (HD5) and an indica rice variety 1892S using the method of bulked segregant analysis coupled with whole-genome sequencing (BSA-seq). HD5 was more tolerant to high temperature than 1892S at the seedling stage. By analyzing a pair of opposite DNA pools made from 124 extremely-sensitive seedlings and 178 extremely-tolerant seedlings from the F2 population using the block regression mapping (BRM) method, we mapped a QTL on chromosome 12, of which the additive effect was estimated to explain 3.75% of the phenotypic variance. We named the QTL qSLHT12.1, which must be a novel QTL, because no QTLs for rice seedling tolerance to heat stress have been mapped on chromosome 12 before. The information obtained in this study will facilitate marker-assisted breeding of heat-resistant lines and positional cloning of the gene conferring heat tolerance in rice.

Similar content being viewed by others
Notes
The author contributed equally to this work.
REFERENCES
Akhter, D., Qin, R., Nath, U.K., et al., A rice gene, OsPL, encoding a MYB family transcription factor confers anthocyanin synthesis, heat stress response and hormonal signaling, Gene, 2019, vol. 699, pp. 62–72. https://doi.org/10.1016/j.gene.2019.03.013
Asako, K., Bao, G., Ye, S., et al., Detection of quantitative trait loci for white-back and basal-white kernels under high temperature stress in Japonica rice varieties, Breed. Sci., 2007, vol. 57, pp. 107–116.
Baliuag, N.A., Redona, E.D., Hernandez, J.E., et al., Genetic analysis for heat tolerance and early morning flowering traits at flowering stage in rice (Oryza sativa L.), Philipp. J. Crop Sci., 2015, vol. 40, pp. 62–72.
Cao, L., Zhu, J., Zhao, S., et al., Mapping QTLs for heat tolerance in a DH population from indica-japonica cross of rice (Oryza sativa L.), J. Agr. Sci.-Cambridge, 2002, vol. 10, pp. 210–214.
Cao, Z., Tang, X., Xiao, W., et al., Identification and genetic effect analysis of QTL (qHTH10) for heat tolerance at heading and flowering stage of rice, MPB, 2019, vol. 17, pp. 2223–2230.
Cao, Z., Li, Y., Tang, H., et al., Fine mapping of the qHTB1-1QTL, which confers heat tolerance at the booting stage, using an Oryza rufipogon Griff. introgression line, Theor. Appl. Genet., 2020, vol. 133, pp. 1161–1175. https://doi.org/10.1007/s00122-020-03539-7
Chen, Q., Yu, S., Li, C., et al., Identification of QTLs for heat tolerance at flowering stage in rice, Sci. Agric. Sin., 2008, vol. 41, pp. 315–321. https://doi.org/10.3864/j.issn.0578-1752.2008.02.001
Chen, L., Wang, Q., Tang, M., et al., QTL mapping and identification of candidate genes for heat tolerance at the flowering stage in rice, Front. Genet., 2021, vol. 11, p. 621871. https://doi.org/10.13271/j.mpb.017.002223
Cheng, L., Wang, J., Uzokwe, V., et al., Genetic analysis of cold tolerance at seedling stage and heat tolerance at anthesis in rice (Oryza sativa L.), J. Integr. Agr., 2012, vol. 11, pp. 359–367. https://doi.org/10.1016/S2095-3119(12)60020-3
Cingolani, P., Platts, A., Wang, L.L., et al., A program for annotating and predicting the effects of single nucleotide polymorphisms, Snp Eff: SNPs in the genome of Drosophila melanogaster strain w 1118; iso-2; iso-3, Fly, 2012, vol. 6, pp. 80–92.
Doyle, J. and Doyle, J., A rapid procedure for DNA purification from small quantities of fresh leaf tissue, Phytochem. Bull., 1987, vol. 19, pp. 11–15.
Driedonks, N., Rieu, I., and Vriezen, W.H., Breeding for plant heat tolerance at vegetative and reproductive stages, Plant Reprod., 2016, vol. 29, pp. 67–79. https://doi.org/10.1007/s00497-016-0275-9
Garrison, E. and Marth, G., Haplotype-based variant detection from short-read sequencing, Quant. Biol., 2012.
Hatfield, J.L. and Prueger, J.H., Temperature extremes: effect on plant growth and development, Weather Clim. Extremes, 2015, vol. 10, pp. 4–10. https://doi.org/10.1016/j.wace.2015.08.001
Hirabayashi, H., Sasaki, K., Kambe, T., et al., qEMF3, a novel QTL for the early-morning flowering trait from wild rice, Oryza officinalis, to mitigate heat stress damage at flowering in rice, O. sativa, J. Exp. Bot., 2015, vol. 66, pp. 1227–1236. https://doi.org/10.1093/jxb/eru474
Huang, L., Tang, W., Bu, S., et al., BRM: a statistical method for QTL mapping based on bulked segregant analysis by deep sequencing, Bioinformatics, 2020, vol. 36, pp. 2150–2156. https://doi.org/10.1093/bioinformatics/btz861
Jagadish, S.V.K., Craufurd, P.P.Q., and Wheeler, T.R., Phenotyping parents of mapping populations of rice for heat tolerance during anthesis, Crop Sci., 2008, vol. 48, pp. 1140–1146. https://doi.org/10.2135/cropsci2007.10.0559
Jagadish, S.V.K., Cairns, J., Lafitte, R., et al., Genetic analysis of heat tolerance at anthesis in rice, Crop Sci., 2010, vol. 50, pp. 1633–1641. https://doi.org/10.2135/cropsci2009.09.0516
Jagadish, S.V.K., Septiningsih, E.M., Kohli, A., et al., Genetic advances in adapting rice to a rapidly changing climate, J. Agro CropSci., 2012, vol. 198, pp. 360–373. https://doi.org/10.1111/j.1439-037X.2012.00525.x
Kawahara, Y., Bastide, M., Hamilton, J.P., et al., Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data, Rice, 2013, vol. 6, p. 4. https://doi.org/10.1186/1939-8433-6-4
Kilasi, N.L., Singh, J., Vallejos, C.E., et al., Heat stress tolerance in rice (Oryza sativa L.): identification of quantitative trait loci and candidate genes for seedling growth under heat stress, Front. Plant Sci., 2018, vol. 9, p. 1578. https://doi.org/10.3389/fpls.2018.01578
Kobayashi, A., Bao, G., Ye, S., et al., Detection of quantitative trait loci for white-back and basal-white kernels under high temperature stress in Japonica rice varieties, Breed. Sci., 2007, vol. 57, pp. 107–116. https://doi.org/10.1270/jsbbs.57.107
Li, H. and Durbin, R., Fast and accurate short read alignment with burrows-wheeler transform, Bioinformatics, 2009, vol. 25, pp. 1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Li, H., Handsaker, B., Wysoker, A., et al., The sequence alignment/map format and SAM tools, Bioinformatics, 2009, vol. 25, pp. 2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Li, M.M., Li, X., Yu, L.Q., et al., Identification of QTLs associated with heat tolerance at the heading and flowering stage in rice (Oryza Sativa L.), Euphytica, 2018, vol. 214, pp. 1–11. https://doi.org/10.1007/s10681-018-2136-0
Liang, T., Chi, W., Huang, L., et al., Bulked segregant analysis coupled with whole-genome sequencing (BSA-seq) mapping identifies a novel pi21 haplotype conferring basal resistance to rice blast disease, Int. J. Mol. Sci., 2020, vol. 21, p. 2162. https://doi.org/10.3390/ijms21062162
Liu, Q., Yang, T., Yu, T., et al., Integrating small RNA sequencing with QTL mapping for identification of miRNAs and their target genes associated with heat tolerance at the flowering stage in rice, Front. Plant Sci., 2017, vol. 8, p. 43. https://doi.org/10.3389/fpls.2017.00043
Magwene, P.M., Willis, J.H., and Kelly, J.K., The statistics of bulk segregant analysis using next generation sequencing, PLoS Comput. Biol., 2011, vol. 7, p. e1002255. https://doi.org/10.1371/journal.pcbi.1002255
Miyahara, K., Wada, T., Sonoda, J.Y., et al., Detection and validation of QTLs for milky-white grains caused by high temperature during the ripening period in japonica rice, Breed. Sci., 2017, vol. 67, pp. 333–339. https://doi.org/10.1270/jsbbs.16203
Murata, K., Iyama, Y., Yamaguchi, T., et al., Identification of a novel gene (Apq1) from the indica rice cultivar ‘Habataki’ that improves the quality of grains produced under high temperature stress, Breed. Sci., 2014, vol. 64, pp. 273–281. https://doi.org/10.1270/jsbbs.64.273
Nishida, S., Dissanayaka, D.M.S.B., Honda, S., et al., Identification of genomic regions associated with low phosphorus tolerance in japonica rice (Oryza sativa L.) by QTL-Seq, J. Soil Sci. Plant Nutr., 2018, vol. 64, pp. 278–281. https://doi.org/10.1080/00380768.2017.1412238
Nubankoh, P., Wanchana, S., Saensuk, C., et al., QTL-seq reveals genomic regions associated with spikelet fertility in response to a high temperature in rice (Oryza sativa L.), Plant Cell Rep., 2020, vol. 39, pp. 149–162. https://doi.org/10.1007/s00299-019-02477-z
Pan, Y., Liang, H., Gao, L., et al., Transcriptomic profiling of germinating seeds under cold stress and characterization of the cold-tolerant gene LTG5 in rice, BMC Biol., 2020, vol. 20, p. 371. https://doi.org/10.1186/s12870-020-02569-z
Park, J.R., Yang, W.T., Kim, D.H., et al., Identification of a novel gene, Osbht, in response to high temperature tolerance at booting stage in rice, Int. J. Mol. Sci., 2020, vol. 21, p. 5862. https://doi.org/10.3390/ijms21165862
Ps, S., Sv, A.M., Prakash, C., et al., High resolution mapping of QTLs for heat tolerance in rice using a 5K SNP array, Rice, 2017, vol. 10, p. 28. https://doi.org/10.1186/s12284-017-0167-0
Solomon, S., Qin, D., Manning, M., et al., Summary for Policymakers, in Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC), 2007.
Tabata, M., Hirabayashi, H., Takeuchi, Y., et al., Mapping of quantitative trait loci for the occurrence of White-Back kernels associated with high temperatures during the ripening period of rice (Oryza sativa L.), Breed. Sci., 2007, vol. 57, pp. 47–52. https://doi.org/10.1270/jsbbs.57.47
Takagi, H., Abe, A., Yoshida, K., et al., QTL-seq: rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations, Plant J., 2013, vol. 74, pp. 174–183. https://doi.org/10.1111/tpj.12105
Tang, W., Huang, L., Bu, S., et al., Estimation of QTL heritability based on pooled sequencing data, Bioinformatics, 2018, vol. 34, pp. 978–984. https://doi.org/10.1093/bioinformatics/btx703
Tazib, T., Kobayashi, Y., Koyama, H., et al., QTL analyses for anther length and dehiscence at flowering as traits for the tolerance of extreme temperatures in rice (Oryza sativa L.), Euphytica, 2015, vol. 203, pp. 629–642. https://doi.org/10.1007/s10681-014-1291-1
Thanh, P.T., Phan, P.D.T., Ishikawa, R., et al., QTL analysis for flowering time using backcross population between Oryza sativa Nipponbare and O. rufipogon, Genes Genet. Syst., 2010, vol. 85, pp. 273–279. https://doi.org/10.1266/ggs.85.273
Wada, T., Miyahara, K., Sonoda, J.Y., et al., Detection of QTLs for white-back and basal-white grains caused by high temperature during ripening period in japonica rice, Breed. Sci., 2015, vol. 65, pp. 216–225. https://doi.org/10.1270/jsbbs.65.216
Waghmare, S.G., Sindhumole, P., Mathew, D., et al., Identification of QTL linked to heat tolerance in rice (Oryza Sativa L.) using SSR markers through bulked segregant analysis, Electron. J. Plant Breed., 2021, vol. 12, pp. 46–53. https://doi.org/10.37992/2021.1201.007
Wei, H., Liu, J., Wang, Y., et al., A dominant major locus in chromosome 9 of rice (Oryza sativa L.) confers tolerance to 48 °C high temperature at seedling stage, J. Hered., 2013, vol. 104, pp. 287–294. https://doi.org/10.1093/jhered/ess103
Welch, J.R., Vincent, J.R., Auffhammer, M., et al., Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures, Proc. Natl. Acad. Sci. U. S. A., 2010, vol. 107, pp. 14562–14567. https://doi.org/10.1073/pnas.1001222107
Xiao, Y., Pan, Y., Luo, L., et al., Quantitative trait loci associated with seed set under high temperature stress at the flowering stage in rice, Euphytica, 2011, vol. 178, pp. 331–338. https://doi.org/10.1007/s10681-010-0300-2
Yan, C., Zhan, G., Hong, X., et al., Identification and fine mapping of a major QTL, TT1-2, that plays significant roles in regulating heat tolerance in rice, Plant Mol. Biol. Rep., 2020, vol. 39, pp. 376–385. https://doi.org/10.1007/s11105-020-01256-5
Yang, Z., Huang, D., Tang, W., et al., Mapping of quantitative trait loci underlying cold tolerance in rice seedlings via high-throughput sequencing of pooled extremes, PloS One, 2013, vol. 8, p. e68433. https://doi.org/10.1371/journal.pone.0068433
Yang, X., Xia, X., Zhang, Z., et al., QTL mapping by whole genome re-sequencing and analysis of candidate genes for nitrogen use efficiency in rice, Front. Plant Sci., 2017, vol. 8, p. 1634. https://doi.org/10.3389/fpls.2017.01634
Ye, C., Argayoso, M.A., Redoña, E.D., et al., Mapping QTL for heat tolerance at flowering stage in rice using SNP markers, Plant Breed., 2012, vol. 131, pp. 33–41. https://doi.org/10.1111/j.1439-0523.2011.01924.x
Ye, C., Tenorio, F.A., Argayoso, M.A., et al., Identifying and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations, BMC Genet., 2015a, vol. 16, pp. 41–50. https://doi.org/10.1186/s12863-015-0199-7
Ye, C., Tenorio, F.A., Redoña, E.D., et al., Fine-mapping and validating qHTSF4.1 to increase spikelet fertility under heat stress at flowering in rice, Theor. Appl. Genet., 2015b, vol. 128, pp. 1507–1517. https://doi.org/10.1007/s00122-015-2526-9
Yoshida, S., Forno, D.A., Cock, J.H., et al., Laboratory manual for physiological studies of rice, Int. Rice Res. Inst. Philipp., 1976, pp. 61–66.
Zhang, C., Chen, F., Hong, R., et al., Mapping QTLs for heat tolerance in rice (Oryza sativa L.) at heading stage using chromosome segment substitution lines, Agric. Biotechnol., 2017, vol. 6, pp. 15–18. https://doi.org/10.19759/j.cnki.2164-4993.2017.02.004
Zhao, L., Lei, J., Huang, Y., et al., Mapping quantitative trait loci for heat tolerance at anthesis in rice using chromosomal segment substitution lines, Breed. Sci., 2016, vol. 66, pp. 358–366. https://doi.org/10.1270/jsbbs.15084
Zheng, J., Li, X., Su, H., et al., Construction of a genetic linkage map and QTL location for heat tolerance in japonica rice resources Rejing35, J. Nuclear Agric. Sci., 2017, vol. 31, pp. 844–851. https://doi.org/10.11869/j.issn.100-8551.2017.05.0844
Zhu, C., Jiang, L., Zhang, W., et al., Identifying QTLs for thermo-tolerance of amylose content and gel consistency in rice, Chin. J. Rice Sci., 2006, vol. 20, pp. 248–252.
Zhu, S., Huang, R., Wai, H.P., et al., Mapping quantitative trait loci for heat tolerance at the booting stage using chromosomal segment substitution lines in rice, Physi-ol. Mol. Biol. Plants, 2017, vol. 23, pp. 817–825. https://doi.org/10.1007/s12298-017-0465-4
ACKNOWLEDGMENTS
We really thank funding bodies:
(1) The grants GJJ180879 funded by the Science and Technology Project of the Education Department of Jiangxi Province.
(2) The grants 2018FJCBD01 funded by the Open Project of the Key Laboratory of Crop Design and Breeding of Fujian Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
About this article
Cite this article
Wu, Y., Wu, H., Zhang, G. et al. Pooled Mapping of Quantitative Trait Loci Conferring Heat Tolerance at Seedling Stage in Rice (Oryza sativa L.). Cytol. Genet. 57, 367–373 (2023). https://doi.org/10.3103/S0095452723040126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452723040126