Abstract
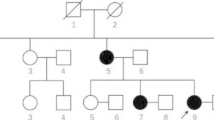
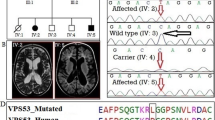
Intellectual disability (ID) is a common neurodevelopmental disorder characterized by significantly impaired adaptive behavior and cognitive capacity. High throughput sequencing approaches have revealed the genetic etiologies for 25–50% of ID patients, while inherited genetic mutations were detected in <5% cases. Here, we investigated the genetic cause for non-syndromic ID in a Han Chinese family. Whole genome sequencing was performed on identical twin sisters diagnosed with ID, their respective children, and their asymptomatic parents. Data was filtered for rare variants, and in silico prediction tools were used to establish pathogenic alleles. Candidate mutations were validated by Sanger sequencing. In silico modeling was used to evaluate the mutation’s effects on the protein encoded by a candidate coding gene. A novel heterozygous variant in the ZBTB18 gene c.1323C>G (p.His441Gln) was identified. This variant co-segregated with affected individuals in an autosomal dominant pattern and was not detected in asymptomatic family members. Molecular studies reveal that a p.His441Gln substitution disrupts zinc binding within the second zinc finger and disrupts the capacity for ZBTB18 to bind DNA. This is the first report of an inherited ZBTB18 mutation for ID. This study further validates WGS for the accurate molecular diagnosis of ID.




Similar content being viewed by others
Data availability
The data and images used in this article are available from the corresponding author on reasonable request.
Abbreviations
- ID:
-
intellectual disability
- CNVs:
-
copy number variants
- NGS:
-
next-generation sequencing
- WES:
-
whole-exome sequencing
- WGS:
-
whole-genome sequencing
- MR:
-
magnetic resonance
- MRI:
-
magnetic resonance imaging
References
Salvador-Carulla L, Reed GM, Vaez-Azizi LM, Cooper SA, Martinez-Leal R, Bertelli M, Adnams C, Cooray S, Deb S, Akoury-Dirani L et al (2011) Intellectual developmental disorders: towards a new name, definition and framework for “mental retardation/intellectual disability” in ICD-11. World Psychiatry 10(3):175–180
Moeschler JB, Shevell M (2014) Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics 134(3):e903–e918
Vissers LE, Gilissen C, Veltman JA (2016) Genetic studies in intellectual disability and related disorders. Nat Rev Genet 17(1):9–18
Vasudevan P, Suri M (2017) A clinical approach to developmental delay and intellectual disability. Clin Med 17(6):558–561
Feldkamp ML, Carey JC, Byrne JLB, Krikov S, Botto LD (2017) Etiology and clinical presentation of birth defects: population based study. BMJ 357:j2249
Srour M, Shevell M (2014) Genetics and the investigation of developmental delay/intellectual disability. Arch Dis Child 99(4):386–389
Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, Albrecht B, Bartholdi D, Beygo J, Di Donato N et al (2012) Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 380(9854):1674–1682
Gilissen C, Hehir-Kwa JY, Thung DT, van de Vorst M, van Bon BW, Willemsen MH, Kwint M, Janssen IM, Hoischen A, Schenck A et al (2014) Genome sequencing identifies major causes of severe intellectual disability. Nature 511(7509):344–347
Srivastava S, Cohen JS, Vernon H, Baranano K, McClellan R, Jamal L, Naidu S, Fatemi A (2014) Clinical whole exome sequencing in child neurology practice. Ann Neurol 76(4):473–483
Farwell Hagman KD, Shinde DN, Mroske C, Smith E, Radtke K, Shahmirzadi L, El-Khechen D, Powis Z, Chao EC, Alcaraz WA et al (2016) Candidate-gene criteria for clinical reporting: diagnostic exome sequencing identifies altered candidate genes among 8% of patients with undiagnosed diseases. Genet Med 19(2):224–235
Bowling KM, Thompson ML, Amaral MD, Finnila CR, Hiatt SM, Engel KL, Cochran JN, Brothers KB, East KM, Gray DE et al (2017) Genomic diagnosis for children with intellectual disability and/or developmental delay. Genome Med 9(1):43
Popp B, Ekici AB, Thiel CT, Hoyer J, Wiesener A, Kraus C, Reis A, Zweier C (2017) Exome Pool-Seq in neurodevelopmental disorders. Eur J Hum Genet 25(12):1364–1376
Lindstrand A, Eisfeldt J, Pettersson M, Carvalho CMB, Kvarnung M, Grigelioniene G, Anderlid BM, Bjerin O, Gustavsson P, Hammarsjo A et al (2019) From cytogenetics to cytogenomics: whole-genome sequencing as a first-line test comprehensively captures the diverse spectrum of disease-causing genetic variation underlying intellectual disability. Genome Med 11(1):68
Thuresson AC, Soussi Zander C, Zhao JJ, Halvardson J, Maqbool K, Mansson E, Stenninger E, Holmlund U, Ohrner Y, Feuk L (2019) Whole genome sequencing of consanguineous families reveals novel pathogenic variants in intellectual disability. Clin Genet 95(3):436–439
Han JY, Lee IG (2020) Genetic tests by next-generation sequencing in children with developmental delay and/or intellectual disability. Clin Exp Pediatr 63(6):195–202
Wang J, Wang Y, Wang L, Chen WY, Sheng M (2020) The diagnostic yield of intellectual disability: combined whole genome low-coverage sequencing and medical exome sequencing. BMC Med Genet 13(1):70
Kochinke K, Zweier C, Nijhof B, Fenckova M, Cizek P, Honti F, Keerthikumar S, Oortveld MA, Kleefstra T, Kramer JM et al (2016) Systematic phenomics analysis deconvolutes genes mutated in intellectual disability into biologically coherent modules. Am J Hum Genet 98(1):149–164
Jarvela I, Maatta T, Acharya A, Leppala J, Jhangiani SN, Arvio M, Siren A, Kankuri-Tammilehto M, Kokkonen H, Palomaki M et al (2021) Exome sequencing reveals predominantly de novo variants in disorders with intellectual disability (ID) in the founder population of Finland. Hum Genet 140(7):1011–1029
Ohtaka-Maruyama C, Hirai S, Miwa A, Takahashi A, Okado H (2012) The 5’-flanking region of the RP58 coding sequence shows prominent promoter activity in multipolar cells in the subventricular zone during corticogenesis. Neuroscience 201:67–84
Aoki K, Meng G, Suzuki K, Takashi T, Kameoka Y, Nakahara K, Ishida R, Kasai M (1998) RP58 associates with condensed chromatin and mediates a sequence-specific transcriptional repression. J Biol Chem 273(41):26698–26704
Baubet V, Xiang C, Molczan A, Roccograndi L, Melamed S, Dahmane N (2012) Rp58 is essential for the growth and patterning of the cerebellum and for glutamatergic and GABAergic neuron development. Development 139(11):1903–1909
de Munnik SA, Garcia-Minaur S, Hoischen A, van Bon BW, Boycott KM, Schoots J, Hoefsloot LH, Knoers NV, Bongers EM, Brunner HG (2014) A de novo non-sense mutation in ZBTB18 in a patient with features of the 1q43q44 microdeletion syndrome. Eur J Hum Genet 22(6):844–846
Lopes F, Barbosa M, Ameur A, Soares G, de Sa J, Dias AI, Oliveira G, Cabral P, Temudo T, Calado E et al (2016) Identification of novel genetic causes of Rett syndrome-like phenotypes. J Med Genet 53(3):190–199
Cohen JS, Srivastava S, Farwell Hagman KD, Shinde DN, Huether R, Darcy D, Wallerstein R, Houge G, Berland S, Monaghan KG et al (2017) Further evidence that de novo missense and truncating variants in ZBTB18 cause intellectual disability with variable features. Clin Genet 91(5):697–707
Depienne C, Nava C, Keren B, Heide S, Rastetter A, Passemard S, Chantot-Bastaraud S, Moutard ML, Agrawal PB, VanNoy G et al (2017) Genetic and phenotypic dissection of 1q43q44 microdeletion syndrome and neurodevelopmental phenotypes associated with mutations in ZBTB18 and HNRNPU. Hum Genet 136(4):463–479
Ehmke N, Karge S, Buchmann J, Korinth D, Horn D, Reis O, Hassler F (2017) A de novo nonsense mutation in ZBTB18 plus a de novo 15q13.3 microdeletion in a 6-year-old female. Am J Med Genet A 173(5):1251–1256
van der Schoot V, de Munnik S, Venselaar H, Elting M, Mancini GMS, Ravenswaaij-Arts CMA, Anderlid BM, Brunner HG, Stevens SJC (2018) Toward clinical and molecular understanding of pathogenic variants in the ZBTB18 gene. Mol Genet Genomic Med 6(3):393–400
Chen Y, Shi C, Huang Z, Zhang Y, Li S, Li Y, Ye J, Yu C, Li Z, Zhang X et al (2018) SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 7(1):1–6
Vasimuddin Md SM, Heng Li, Srinivas Aluru: Efficient architecture-aware acceleration of BWA-MEM for multicore systems. IEEE International Parallel and Distributed Processing Symposium (IPDPS), Rio de Janeiro, Brazil, 2019:pp. 314-324.
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M et al (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43(5):491–498
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F (2016) The Ensembl variant effect predictor. Genome Biol 17(1):122
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR (2015) A global reference for human genetic variation. Nature 526(7571):68–74
Ng PC, Henikoff S (2003) SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31(13):3812–3814
Adzhubei I, Jordan DM, Sunyaev SR (2013) Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet Chapter 7:Unit7 20
Choi Y, Chan AP (2015) PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31(16):2745–2747
Steinhaus R, Proft S, Schuelke M, Cooper DN, Schwarz JM, Seelow D (2021) MutationTaster2021. Nucleic Acids Res 49(W1):W446–W451
Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M (2019) CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res 47(D1):D886–D894
Hemming IA, Clement O, Gladwyn-Ng IE, Cullen HD, Ng HL, See HB, Ngo L, Ulgiati D, Pfleger KDG, Agostino M et al (2019) Disease-associated missense variants in ZBTB18 disrupt DNA binding and impair the development of neurons within the embryonic cerebral cortex. Hum Mutat 40(10):1841–1855
Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abeysinghe S, Krawczak M, Cooper DN (2003) Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 21(6):577–581
Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Jang W et al (2018) ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res 46(D1):D1062–D1067
Hirai S, Miwa A, Ohtaka-Maruyama C, Kasai M, Okabe S, Hata Y, Okado H (2012) RP58 controls neuron and astrocyte differentiation by downregulating the expression of Id1-4 genes in the developing cortex. EMBO J 31(5):1190–1202
Xiang C, Baubet V, Pal S, Holderbaum L, Tatard V, Jiang P, Davuluri RV, Dahmane N (2012) RP58/ZNF238 directly modulates proneurogenic gene levels and is required for neuronal differentiation and brain expansion. Cell Death Differ 19(4):692–702
Ohtaka-Maruyama C, Hirai S, Miwa A, Heng JI, Shitara H, Ishii R, Taya C, Kawano H, Kasai M, Nakajima K et al (2013) RP58 regulates the multipolar-bipolar transition of newborn neurons in the developing cerebral cortex. Cell Rep 3(2):458–471
Heng JI, Qu Z, Ohtaka-Maruyama C, Okado H, Kasai M, Castro D, Guillemot F, Tan SS (2015) The zinc finger transcription factor RP58 negatively regulates Rnd2 for the control of neuronal migration during cerebral cortical development. Cereb Cortex 25(3):806–816
Visich A, Zielenski J, Castanos C, Diez G, Grenoville M, Segal E, Barreiro C, Tsui LC, Chertkoff L (2002) Complete screening of the CFTR gene in Argentine cystic fibrosis patients. Clin Genet 61(3):207–213
Dork T, Dworniczak B, Aulehla-Scholz C, Wieczorek D, Bohm I, Mayerova A, Seydewitz HH, Nieschlag E, Meschede D, Horst J et al (1997) Distinct spectrum of CFTR gene mutations in congenital absence of vas deferens. Hum Genet 100(3-4):365–377
Johnson B, Lowe GC, Futterer J, Lordkipanidze M, MacDonald D, Simpson MA, Sanchez-Guiu I, Drake S, Bem D, Leo V et al (2016) Whole exome sequencing identifies genetic variants in inherited thrombocytopenia with secondary qualitative function defects. Haematologica 101(10):1170–1179
Kwon JA, Lee SY, Ahn EK, Seol SY, Kim MC, Kim SJ, Kim SI, Chu IS, Leem SH (2010) Short rare MUC6 minisatellites-5 alleles influence susceptibility to gastric carcinoma by regulating gene. Hum Mutat 31(8):942–949
Lim ET, Uddin M, De Rubeis S, Chan Y, Kamumbu AS, Zhang X, D'Gama AM, Kim SN, Hill RS, Goldberg AP et al (2017) Rates, distribution and implications of postzygotic mosaic mutations in autism spectrum disorder. Nat Neurosci 20(9):1217–1224
Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, Rippey C, Shahin H, Nimgaonkar VL, Go RC et al (2013) Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 154(3):518–529
Nagamani SC, Erez A, Bay C, Pettigrew A, Lalani SR, Herman K, Graham BH, Nowaczyk MJ, Proud M, Craigen WJ et al (2012) Delineation of a deletion region critical for corpus callosal abnormalities in chromosome 1q43-q44. Eur J Hum Genet 20(2):176–179
Ballif BC, Rosenfeld JA, Traylor R, Theisen A, Bader PI, Ladda RL, Sell SL, Steinraths M, Surti U, McGuire M et al (2012) High-resolution array CGH defines critical regions and candidate genes for microcephaly, abnormalities of the corpus callosum, and seizure phenotypes in patients with microdeletions of 1q43q44. Hum Genet 131(1):145–156
Perlman SJ, Kulkarni S, Manwaring L, Shinawi M (2013) Haploinsufficiency of ZNF238 is associated with corpus callosum abnormalities in 1q44 deletions. Am J Med Genet A 161A(4):711–716
JF MR, Clayton S, Fitzgerald TW, Kaplanis J (2017) Prigmore E: Prevalence and architecture of de novo mutations in developmental disorders. Nature 542(7642):433–438
Evers C, Staufner C, Granzow M, Paramasivam N, Hinderhofer K, Kaufmann L, Fischer C, Thiel C, Opladen T, Kotzaeridou U et al (2017) Impact of clinical exomes in neurodevelopmental and neurometabolic disorders. Mol Genet Metab 121(4):297–307
Kosmicki JA, Samocha KE, Howrigan DP, Sanders SJ, Slowikowski K, Lek M, Karczewski KJ, Cutler DJ, Devlin B, Roeder K et al (2017) Refining the role of de novo protein-truncating variants in neurodevelopmental disorders by using population reference samples. Nat Genet 49(4):504–510
Trinh J, Kandaswamy KK, Werber M, Weiss MER, Oprea G, Kishore S, Lohmann K, Rolfs A (2019) Novel pathogenic variants and multiple molecular diagnoses in neurodevelopmental disorders. J Neurodev Disord 11(1):11
Vetrini F, McKee S, Rosenfeld JA, Suri M, Lewis AM, Nugent KM, Roeder E, Littlejohn RO, Holder S, Zhu W et al (2019) De novo and inherited TCF20 pathogenic variants are associated with intellectual disability, dysmorphic features, hypotonia, and neurological impairments with similarities to Smith-Magenis syndrome. Genome Med 11(1):12
Castel SE, Cervera A, Mohammadi P, Aguet F, Reverter F, Wolman A, Guigo R, Iossifov I, Vasileva A, Lappalainen T (2018) Modified penetrance of coding variants by cis-regulatory variation contributes to disease risk. Nat Genet 50(9):1327–1334
Sherry ST, Ward M, Sirotkin K (1999) dbSNP-database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res 9(8):677–679
Hemming IA, Blake S, Agostino M, Heng JI (2020) General population ZBTB18 missense variants influence DNA binding and transcriptional regulation. Hum Mutat 41(9):1629–1644
Acknowledgements
We are very grateful to the family members for their participation in this study.
Funding
This study was supported by the National Key Research and Development Program of China (2022YFC2703700, 2022YFC2703702) and the Program for Changjiang Scholars and Innovative Research Team in University (ID: IRT0935).
Author information
Authors and Affiliations
Contributions
Nana Li and Ping Yu developed the study design. Nana Li, Hong Kang, Julian Heng, and Ping Yu conducted the experiment and drafted the manuscript. Zhen Liu, Ying Deng, Meixian Wang, and Lu Li assisted in analyzing the genomic data. Yanna Zou, Hong Qin, and Xiaoqiong Qiu participated in the clinical evaluation of the patients. Mark Agostino and Julian I-T Heng performed molecular modeling studies. Yanping Wang, Jun Zhu, and Julian I-T Heng participated in the critical review and revision of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(XLSX 9 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, N., Kang, H., Zou, Y. et al. A novel heterozygous ZBTB18 missense mutation in a family with non-syndromic intellectual disability. Neurogenetics 24, 251–262 (2023). https://doi.org/10.1007/s10048-023-00727-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-023-00727-7