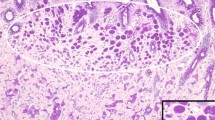
Abstract
Pathogenic germline variants (PGVs) in the CDH1 gene are associated with diffuse gastric and lobular breast cancer syndrome (DGLBC) and can increase the lifetime risk for both diffuse gastric cancer and lobular breast cancer. Given the risk for diffuse gastric cancer among individuals with CDH1 PGVs is up to 30–40%, prophylactic total gastrectomy is often recommended to affected individuals. Therefore, accurate interpretation of CDH1 variants is of the utmost importance for proper clinical decision-making. Herein we present a 45-year-old female, with lobular breast cancer and a father with gastric cancer of unknown pathology at age 48, who was identified to have an intronic variant of uncertain significance in the CDH1 gene, specifically c.833-9 C > G. Although the proband did not meet the International Gastric Cancer Linkage Consortium (IGCLC) criteria for gastric surveillance, she elected to pursue an upper endoscopy where non-targeted gastric biopsies identified a focus of signet ring cell carcinoma (SRCC). The proband then underwent a total gastrectomy, revealing numerous SRCC foci, but no invasive diffuse gastric cancer. Simultaneously, a genetic testing laboratory performed RNA sequencing to further analyze the CDH1 intronic variant, identifying an abnormal transcript from a novel acceptor splice site. The RNA analysis in conjunction with the patient’s gastric foci of SRCC and family history was sufficient evidence for reclassification of the variant from uncertain significance to likely pathogenic. In conclusion, we report the first case of the CDH1 c.833-9 C > G intronic variant being associated with DGLBC and illustrate how collaboration among clinicians, laboratory personnel, and patients is crucial for variant resolution.


Similar content being viewed by others
References
Blair VR, McLeod M, Carneiro F et al (2020) Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 21:e386–e397. https://doi.org/10.1016/S1470-2045(20)30219-9
Hansford S, Kaurah P, Li-Chang H et al (2015) Hereditary diffuse gastric Cancer syndrome: CDH1 mutations and Beyond. JAMA Oncol 1:23–32. https://doi.org/10.1001/jamaoncol.2014.168
Lobo S, Benusiglio PR, Coulet F et al (2021) Cancer predisposition and germline CTNNA1 variants. Eur J Med Genet 64:104316. https://doi.org/10.1016/j.ejmg.2021.104316
Clark DF, Michalski ST, Tondon R et al (2020) Loss-of-function variants in CTNNA1 detected on multigene panel testing in individuals with gastric or breast cancer. Genet Med 22:840–846. https://doi.org/10.1038/s41436-020-0753-1
Pharoah PD, Guilford P, Caldas C, International Gastric Cancer Linkage Consortium (2001) Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 121:1348–1353. https://doi.org/10.1053/gast.2001.29611
Xicola RM, Li S, Rodriguez N et al (2019) Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet 56:838–843. https://doi.org/10.1136/jmedgenet-2019-105991
Roberts ME, Ranola JMO, Marshall ML et al (2019) Comparison of CDH1 Penetrance estimates in clinically ascertained families vs families ascertained for multiple gastric cancers. JAMA Oncol 5:1325–1331. https://doi.org/10.1001/jamaoncol.2019.1208
Lowstuter K, Espenschied CR, Sturgeon D et al (2017) Unexpected CDH1 mutations identified on Multigene Panels Pose Clinical Management Challenges. JCO Precis Oncol 1:1–12. https://doi.org/10.1200/PO.16.00021
Katona BW, Clark DF, Domchek SM (2020) CDH1 on Multigene Panel Testing: look before you Leap. J Natl Cancer Inst 112:330–334. https://doi.org/10.1093/jnci/djz229
Daly MB et al (2023) PTAZ. National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic.
Manahan ER, Kuerer HM, Sebastian M, Hughes KS, Boughey JC, Euhus DM, Boolbol SK, Taylor WA (2019) Consensus Guidelines on Genetic` testing for Hereditary breast Cancer from the american society of breast surgeons. Ann Surg Oncol 26:3025–3031. https://doi.org/10.1245/s10434-019-07549-8
Federici G, Soddu S (2020) Variants of uncertain significance in the era of high-throughput genome sequencing: a lesson from breast and ovary cancers. J Experimental Clin Cancer Res 39:46. https://doi.org/10.1186/s13046-020-01554-6
Idos GE, Kurian AW, Ricker C et al (2019) Multicenter prospective cohort study of the Diagnostic yield and patient experience of Multiplex Gene Panel Testing for Hereditary Cancer Risk. https://doi.org/10.1200/po.18.00217. JCO Precis Oncol
Mighton C, Shickh S, Uleryk E et al (2021) Clinical and psychological outcomes of receiving a variant of uncertain significance from multigene panel testing or genomic sequencing: a systematic review and meta-analysis. Genet Sci 23:22–33. https://doi.org/10.1038/s41436-020-00957-2
Lerner BA, Xicola RM, Rodriguez NJ, Karam R, Llor X (2023) Simplified and more sensitive criteria for identifying individuals with pathogenic CDH1 variants. J Med Genet 60:36–40. https://doi.org/10.1136/jmedgenet-2021-108169
Rofes P, Menéndez M, González S et al (2020) Improving genetic testing in Hereditary Cancer by RNA analysis: tools to prioritize Splicing Studies and Challenges in applying American College of Medical Genetics and Genomics Guidelines. J Mol Diagn 22:1453–1468. https://doi.org/10.1016/j.jmoldx.2020.09.007
Karam R, Conner B, LaDuca H et al (2019) Assessment of Diagnostic Outcomes of RNA genetic testing for Hereditary Cancer. JAMA Netw Open 2:e1913900. https://doi.org/10.1001/jamanetworkopen.2019.13900
Scotti MM, Swanson MS (2016) RNA mis-splicing in disease. Nat Rev Genet 17:19–32. https://doi.org/10.1038/nrg.2015.3
Rhine CL, Cygan KJ, Soemedi R et al (2018) Hereditary cancer genes are highly susceptible to splicing mutations. PLoS Genet 14:e1007231. https://doi.org/10.1371/journal.pgen.1007231
Barbosa-Matos R, Silva L, Garrido R, L. et al (2021) The CDH1 c.1901 C > T variant: a founder variant in the Portuguese Population with severe impact in mRNA splicing. Cancers 13:4464. https://doi.org/10.3390/cancers13174464
Kaurah P, MacMillan A, Boyd N et al (2007) Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 297(21):2360–2372. https://doi.org/10.1001/jama.297.21.2360
Gamble LA, Rossi A, Fasaye GA et al (2022) Association between Hereditary lobular breast Cancer due to CDH1 variants and gastric Cancer risk. JAMA Surg 157:18–22. https://doi.org/10.1001/jamasurg.2021.5118
Garcia-Pelaez J, Barbosa-Matos R, Lobo S et al (2023) Genotype-first approach to identify associations between CDH1 germline variants and cancer phenotypes: a multicentre study by the european Reference Network on genetic Tumour Risk Syndromes. Lancet Oncol 1:91–106. https://doi.org/10.1016/S1470-2045(22)00643-X
Lecuit T, Yap AS (2015) E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol 17:533–539. https://doi.org/10.1038/ncb3136
Oliveira C, Senz J, Kaurah P et al (2009) Germline CDH1 deletions in hereditary diffuse gastric cancer families. Hum Mol Genet 18:1545–1555. https://doi.org/10.1093/hmg/ddp046
Lee K, Krempely K, Roberts ME, Anderson MJ et al (2018) Specifications of the ACMG/AMP variant curation guidelines for the analysis of germline CDH1 sequence variants. Hum Mutat 39:1553–1568. https://doi.org/10.1002/humu.23650
Luo X, Maciaszek JL, Thompson BA et al (2023) ClinGen CDH1 variant Curation Expert Panel. Optimising clinical care through CDH1-specific germline variant curation: improvement of clinical assertions and updated curation guidelines. J Med Genet 60:568–575. https://doi.org/10.1136/jmg-2022-108807
Horton C, Cass A, Conner BR et al (2022) Mutational and splicing landscape in a cohort of 43,000 patients tested for hereditary cancer. NPJ Genom Med 7:49. https://doi.org/10.1038/s41525-022-00323-y
Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF et al (2019) Predicting Splicing from primary sequence with deep learning. Cell 176:535–548e24. https://doi.org/10.1016/j.cell.2018.12.015
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet medicine: official J Am Coll Med Genet 17:405–424. https://doi.org/10.1038/gim.2015.30
Karczewski KJ, Francioli LC, Tiao G et al (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581:434–443. https://doi.org/10.1038/s41586-020-2308-7
Asif B, Sarvestani AL, Gamble LA, Samaranayake SG, Famiglietti AL, Fasaye GA, Quezado M, Miettinen M, Korman L, Koh C, Heller T, Davis JL (2023) Cancer surveillance as an alternative to prophylactic total gastrectomy in hereditary diffuse gastric cancer: a prospective cohort study. Lancet Oncol 24:383–391. https://doi.org/10.1016/S1470-2045(23)00057-8
Lee CYC, Olivier A, Honing J, Lydon AM, Richardson S, O’Donovan M, Tischkowitz M, Fitzgerald RC, di Pietro M (2023) Endoscopic surveillance with systematic random biopsy for the early diagnosis of hereditary diffuse gastric cancer: a prospective 16-year longitudinal cohort study. Lancet Oncol 24:107–116. https://doi.org/10.1016/S1470-2045(22)00700-8
Henrie A, Hemphill SE, Ruiz-Schultz N et al (2018) ClinVar Miner: demonstrating utility of a web-based tool for viewing and filtering ClinVar data. Hum Mutat 39:1051–1060. https://doi.org/10.1002/humu.23555
Mersch J, Brown N, Pirzadeh-Miller S et al (2018) Prevalence of variant reclassification following hereditary cancer genetic testing. JAMA - Journal of the American Medical Association 320:1266–1274. https://doi.org/10.1001/jama.2018.13152
Slavin TP, Manjarrez S, Pritchard CC et al (2019) The effects of genomic germline variant reclassification on clinical cancer care. Oncotarget 10:417–423. https://doi.org/10.18632/oncotarget.26501
Casaletto J, Cline M, Shirts B (2023) Modeling the impact of data sharing on variant classification. J Am Med Inform Assoc 30:466–474. https://doi.org/10.1093/jamia/ocac232
Vadaparampil ST, Scherr CL, Cragun D et al (2015) Pre-test genetic counseling services for hereditary breast and ovarian cancer delivered by non-genetics professionals in the state of Florida. Clin Genet 87:473–477. https://doi.org/10.1111/cge.12405
Bensend TA, Veach PMC, Niendorf KB (2014) What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns 23:48–63. https://doi.org/10.1007/s10897-013-9605-3
Brierley KL, Campfield D, Ducaine W et al (2010) Errors in delivery of cancer genetics services: implications for practice. Conn Med 74:413–423
Pal T, Cragun D, Lewis C et al (2013) A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers 17:367–375. https://doi.org/10.1089/gtmb.2012.0381
Donohue KE, Gooch C, Katz A et al (2021) Pitfalls and challenges in genetic test interpretation: an exploration of genetic professionals experience with interpretation of results. Clin Genet 99:638–649. https://doi.org/10.1111/cge.13917
Funding
DeGregorio Family Foundation Grant Award (BWK).
Author information
Authors and Affiliations
Contributions
CF, AA, BM, MD, CH, CP, JL, RT, TB, BWK wrote the main manuscript text. TB, RT, BWK prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
CH, CP, and TB are full time employees at Ambry Genetics. The remaining authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corrine Fillman and Arravinth Anantharajah contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fillman, C., Anantharajah, A., Marmelstein, B. et al. Combining clinical and molecular characterization of CDH1: a multidisciplinary approach to reclassification of a splicing variant. Familial Cancer 22, 521–526 (2023). https://doi.org/10.1007/s10689-023-00346-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-023-00346-z