Abstract
Background
Subcutaneous implantable cardioverter–defibrillators (S-ICDs) have been shown to be non-inferior to transvenous ICDs in the prevention of sudden cardiac death (SCD), but there is still a lack of evidence from clinical trials in China. We investigated whether S‑ICD implantation in the Chinese population is safe and feasible and should be promoted in the future.
Methods
Consecutive patients undergoing S‑ICD implantation at our center were enrolled in this retrospective study. Data were collected within the median follow-up period of 554 days. Data concerning patient selection, implantation procedures, complications, and episodes of shock were analyzed.
Results
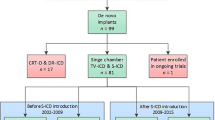
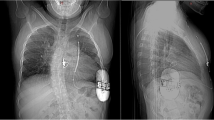
In total, 70.2% of all 47 patients (median age = 39 years) were included for secondary prevention of SCD with different etiologies. Vector screening showed that 98% of patients were with > 1 appropriate vector in all postures. An intraoperative defibrillation test was not performed on six patients because of the high risk of disease deterioration, while all episodes of ventricular fibrillation induced post implantation were terminated by one shock. As expected, no severe complications (e.g., infection and device-related complications) were observed, except for one case of delayed healing of the incision. Overall, 15 patients (31.9%) experienced appropriate shocks (AS) with all episodes terminated by one shock. Two patients (4.3%) experienced inappropriate shocks (IAS) due to noise oversensing, resulting in a high Kaplan–Meier IAS-free rate of 95.7%.
Conclusion
Based on appropriate patient selection and standardized implantation procedures, this real-world study confirmed the safety and efficacy of S‑ICD in Chinese patients, indicating that it may help to promote the prevention of SCD in China.
Zusammenfassung
Hintergrund
Subkutane implantierbare Kardioverter-Defibrillatoren (S-ICD) haben sich gegenüber transvenösen ICD bei der Prävention des plötzlichen Herztods (SCD) als nichtunterlegen erwiesen, aber es fehlt bislang noch Evidenz aus klinischen Studien in China. Die Autoren untersuchten, ob die S‑ICD-Implantation in der chinesischen Bevölkerung sicher und praktikabel ist und ob sie in Zukunft gefördert werden sollte.
Methoden
Konsekutiv sich zur S‑ICD-Implantation vorstellende Patienten im Zentrum der Autoren wurden in die vorliegende retrospektive Studie einbezogen. Für eine mittlere Nachbeobachtungsdauer von 554 Tagen wurden dabei Daten erhoben. Ausgewertet wurden die Daten zur Patientenselektion, zu den Implantationsverfahren, zu Komplikationen und Schockereignissen.
Ergebnisse
Zur Sekundärprävention des SCD unterschiedlicher Ätiologie wurden 70,2 % aller 47 Patienten (mittleres Alter: 39 Jahre) in die Studie eingeschlossen. Das Vektorscreening zeigte, dass 98 % der Patienten > 1 entsprechenden Vektor in allen Positionen aufwiesen. Ein intraoperativer Defibrillationstest wurde bei 6 Patienten wegen des hohen Risikos einer Krankheitsverschlechterung nicht durchgeführt, während alle Phasen von Kammerflimmern, die nach Implantation auftraten, durch einen Schock beendet wurden. Wie erwartet, wurden keine schweren Komplikationen (z. B. Infektionen und gerätbezogene Komplikationen) beobachtet, außer in einem Fall eine verzögerte Abheilung der Inzisionsstelle. Bei 15 Patienten (31,9 %) wurden adäquate Schocks (AS) ausgelöst, wobei alle Phasen durch einen Schock beendet wurden. In 2 Fällen (4,3 %) kam es zu inadäquaten Schocks (IAS) aufgrund von Oversensing, was zu einer hohen IAS-freien Rate nach Kaplan-Meier von 95,7 % führte.
Schlussfolgerung
Auf der Grundlage einer entsprechenden Patientenselektion und standardisierter Implantationsverfahren bestätigte die vorliegende Real-World-Studie die Sicherheit und Wirksamkeit von S‑ICD bei chinesischen Patienten als Hinweis darauf, dass diese Methode möglicherweise zur Förderung der Prävention des SCD in China beitragen kann.




Similar content being viewed by others
References
Miller JD, Yousuf O, Berger RD (2015) The implantable cardioverter-defibrillator: an update. Trends Cardiovasc Med 25:606–611
Burri H, Starck C, Auricchio A et al (2021) EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: endorsed by the heart rhythm society (HRS), the asia pacific heart rhythm society (APHRS), and the latin-American heart rhythm society (LAHRS). Europace 23:983–1008
Koneru JN, Jones P, Hammill EF et al (2018) Risk factors and temporal trends of complications associated with transvenous Implantable cardiac defibrillator leads. J Am Heart Assoc 7:e7691
Knops RE, Olde Nordkamp L, Delnoy PHM et al (2020) Subcutaneous or transvenous defibrillator therapy. N Engl J Med 383:526–536
Gold MR, Lambiase P, El-Chami MF et al (2021) Primary results from the understanding outcomes with the S‑ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 143:7–17
Lambiase PD, Theuns D, Murgatroyd F et al (2022) Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study. Eur Heart J. https://doi.org/10.1093/eurheartj/ehab921
Khanra D, Hamid A, Patel P et al (2022) A real-world experience of subcutaneous and transvenous implantable cardiac defibrillators-comparison with the PRAETORIAN study. J Arrhythm 38:199–212
Knops RE, van der Stuijt W, Delnoy PPHM et al (2022) Efficacy and safety of appropriate shocks and antitachycardia pacing in transvenous and subcutaneous implantable defibrillators: analysis of all appropriate therapy in the PRAETORIAN trial. Circulation 145:321–329
Bögeholz N, Pauls P, Güner F et al (2018) Direct comparison of the novel automated screening tool (AST) versus the manual screening tool (MST) in patients with already implanted subcutaneous ICD. Int J Cardiol 265:90–96
Schukro C, Santer D, Prenner G et al (2020) State-of-the-art consensus on non-transvenous implantable cardioverter-defibrillator therapy. Clin Cardiol 43:1084–1092
Tachibana M, Nishii N, Banba K et al (2019) SMART pass will prevent inappropriate operation of S‑ICD. J Arrhythm 35:86–91
Priori SG, Blomström-Lundqvist C, Mazzanti A et al (2015) 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: association for European paediatric and congenital cardiology (AEPC). Eur Heart J 36:2793–2867
Nishinarita R, Kishihara J, Matsuura G et al (2019) Early inappropriate shock in a subcutaneous cardiac defibrillator due to subcutaneous air. J Arrhythm 35:682–684
Barnett A, Eckhardt L, Leal MA (2018) Seal plug damage causing inappropriate detection and therapy in a subcutaneous defibrillator system. HeartRhythm Case Rep 5:66–69
Nso N, Nassar M, Lakhdar S et al (2022) Comparative assessment of transvenous versus subcutaneous Implantable cardioverter-defibrillator therapy outcomes: an updated systematic review and meta-analysis. Int J Cardiol 349:62–78
Rordorf R, Casula M, Pezza L et al (2021) Subcutaneous versus transvenous implantable defibrillator: an updated meta-analysis. Heart Rhythm 18:382–391
Su L, Guo J, Hao Y, Tan H (2021) Comparing the safety of subcutaneous versus transvenous ICDs: a meta-analysis. J Interv Card Electrophysiol 60:355–363
Basu-Ray I, Liu J, Jia X et al (2017) Subcutaneous versus transvenous Implantable defibrillator therapy: a meta-analysis of case-control studies. JACC Clin Electrophysiol 3:1475–1483
Shu Z (2015) Sudden cardiac death in China: current status and future perspectives. Europace 2:14–18
Vicentini A, Bisignani G, De Vivo S et al (2022) Patient acceptance of subcutaneous versus transvenous defibrillator systems: a multi-center experience. J Cardiovasc Electrophysiol 33:81–89
Okabe T, Miller A, Koppert T et al (2020) Feasibility and safety of same day subcutaneous defibrillator implantation and send home (DASH) strategy. J Interv Card Electrophysiol 52:311–318
Frommeyer G, Zumhagen S, Dechering DG et al (2016) Intraoperative defibrillation testing of subcutaneous implantable cardioverter-defibrillator systems—a simple issue? J Am Heart Assoc 5:e3181
Rudic B, Tülümen E, Fastenrath F et al (2020) Defibrillation failure in patients undergoing replacement of subcutaneous defibrillator pulse generator. Heart Rhythm 17:455–459
Liang JJ, Okamura H, Asirvatham R et al (2019) Comparative outcomes of subcutaneous and transvenous cardioverter-defibrillators. Chin Med J (Engl) 132:631–637
Quast ABE, Baalman S, Brouwer TF et al (2019) A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable cardioverter-defibrillator: the PRAETORIAN score. Heart Rhythm 16:403–410
Al-Ghamdi B, Shafquat A, Alruwaili N et al (2017) Subcutaneous implantable cardioverter defibrillators implantation without defibrillation threshold testing: a single center experience. Cardiol Res 8:319–326
Burke MC, Aasbo J, El-Chami MF et al (2020) 1‑year prospective evaluation of clinical outcomes and shocks: the subcutaneous ICD post approval study. JACC Clin Electrophysiol 6:1537–1550
Quast ABE, Baalman S, Betts TR et al (2019) Rationale and design of the PRAETORIAN-DFT trial: a prospective randomized comparative trial of SubcutanEous Implantable CardiOverter-DefibrillatoR ImplANtation with and without defibrillation testing. Am Heart J 214:167–174
Chen CF, Jin C, Liu MJ, Xu YZ (2019) Efficacy, safety, and in-hospital outcomes of subcutaneous versus transvenous implantable defibrillator therapy: a meta-analysis and systematic review. Medicine 98:e15490
León Salas B, Trujillo‐Martín M, García García J et al (2019) Subcutaneous implantable cardioverter-defibrillator in primary and secondary prevention of sudden cardiac death: a meta-analysis. Pacing Clin Electrophysiol 42:1253–1268
Francia P, Biffi M, Adduci C et al (2020) Implantation technique and optimal subcutaneous defibrillator chest position: a PRAETORIAN score-based study. Europace 22:1822–1829
Sheldon SH, Cunnane R, Lavu M et al (2018) Perioperative hematoma with subcutaneous ICD implantation: Impact of anticoagulation and antiplatelet therapies. Pacing Clin Electrophysiol 41:799–806
Evenson C, Saour B, Afzal MR et al (2019) Increased risk of hematoma with uninterrupted warfarin in patients undergoing implantation of subcutaneous implantable cardioverter defibrillator. Pacing Clin Electrophysiol 42:1111–1114
Jan S (2020) The subcutaneous ICD for prevention of sudden cardiac death: current evidence and future directions. Pacing Clin Electrophysiol 43:1421–1427
Abdin A, Aktaa S (2022) Subcutaneous ICD for more and transvenous ICD for few?! Clin Res Cardiol. https://doi.org/10.1007/s00392-022-01990-8
Nordkamp OLR, Dabiri Abkenari L, Boersma LV et al (2012) The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol 60:1933–1939
Lambiase PD, Barr C, Theuns DA et al (2014) Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S‑ICD registry. Eur Heart J 35:1657–1665
Kooiman KM, Knops R, Olde Nordkamp L et al (2014) Inappropriate subcutaneous implantable cardioverter-defibrillator shocks due to T‑wave oversensing can be prevented: implications for management. Heart Rhythm 11:426–434
Knops RE, Brouwer T, Barr CS et al (2016) The learning curve associated with the introduction of the subcutaneous implantable defibrillator. Europace 18:1010–1005
Funding
Sources: This study was supported by the National Key Clinical Specialty Discipline Construction Program of China (No. YW2021-002), the Shanghai Clinical Research Center for Interventional Medicine (No. 19MC1910300), and the Science and Technology Commission of Shanghai Municipality (No. 19441906500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
L. Zhang, X. Li, Y. Liang, J. Wang, M. Li, L. Pan, X. Chen, S. Qin, J. Bai, W. Wang, Y. Su and J. Ge declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
The authors Lei Zhang, Xiao Li, and Yixiu Liang contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Zhang, L., Li, X., Liang, Y. et al. Real-world evidence for the use of subcutaneous implantable cardioverter–defibrillators in China: A single-center experience. Herz 48, 462–469 (2023). https://doi.org/10.1007/s00059-023-05192-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-023-05192-4
Keywords
- Subcutaneous implantable cardioverter–defibrillator
- Sudden cardiac death
- Efficacy
- Safety
- Chinese population