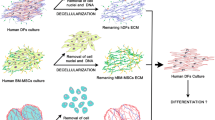
Abstract
The development of cell-culture methods in 3D systems is important and necessary for the development of significant areas of modern cell biology. When cultivating in a 3D system, the tissue-specific architecture is reproduced, with the real microenvironment and cell behavior being more accurately recreated in vivo. Human mesenchymal stem/stromal cells (MSCs) are usually isolated and cultured as a monolayer 2D culture. In this work, we have developed a method for three-dimensional culturing and tissue-specific decidual differentiation of MSCs isolated from human endometrial tissue using a matrix obtained from a decellularized apple. Decellularized apple matrices have sufficient mechanical strength; are biocompatible, affordable, and easy to use; and have ample possibilities for surface modification. This system of cell culturing is suitable for studies using both confocal microscopy and flow cytometry. The model we have developed can become the basis for creating new cell products and tissue engineering structures for the needs of regenerative biomedicine.







Similar content being viewed by others
REFERENCES
Bartosh, T.J. and Ylostalo, J.H., Efficacy of 3D culture priming is maintained in human mesenchymal stem cells after extensive expansion of the cells, Cells, 2019, vol. 8, p. 1031. https://doi.org/10.3390/cells8091031
Baruffaldi, D., Palmara, G., Pirri, C., and Frascella, F., 3D cell culture: recent development in materials with tunable stiffness, ACS Appl. Bio Mater., 2021, vol. 4, p. 2233. https://doi.org/10.1021/acsabm.0c01472
Bilirgen, A.C., Toker, M., Odabas, S., Yetisen, A.K., Garipcan, B., and Tasoglu, S., Plant-based scaffolds in tissue engineering, ACS Biomater. Sci. Eng., 2021, vol. 7, p. 926. https://doi.org/10.1021/acsbiomaterials.0c01527
Bou-Ghannam, S., Kim, K., and Grainger, D.W., 3D cell sheet structure augments mesenchymal stem cell cytokine production, Sci Rep., 2021, vol. 11, p. 8170. https://doi.org/10.1038/s41598-021-87571-7
Chen, G., Qi, Y., Niu, L., DI, T., Zhong, J., Fang, T., and Yan, W., Application of the cell sheet technique in tissue engineering, Biomed. Rep., 2015, vol. 3, p. 749. https://doi.org/10.3892/br.2015.522
Cherng, J.-H., Chou, S.-C., Chen, C.-L., Wang, Y.-W., Chang, S.-J., Fan, G.-Y., Leung, F.-S., and Meng, E., Bacterial cellulose as a potential bio-scaffold for effective re-epithelialization, Ther. Pharm., 2021, vol. 13, p. 1592. https://doi.org/10.3390/pharmaceutics13101592
Domnina, A.P., Novikova, P.V., Fridlyanskaya, I.I., Shilina, M.A., Zenin, V.V., and Nikolsky, N.N., Induction of decidual differentiation of endometrial mesenchymal stem cells, Tsitologiya, 2016, vol. 57, no. 12, p. 880.
Domnina, A.P., Novikova, P.V., Obidina, J.I., Fridlyanskaya, I.I., Alekseenko, L.L., Kozhukharova, I.V., Lyublinskaya, O.G., Zenin, V.V., and Nikolsky, N.N., Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium, Stem Cell Res. Ther., 2018, vol. 9, p. 1. https://doi.org/10.1186/s13287-018-0801-9
Domnina, A.P., Ivanova, J.V., Alekseenko, L.L., Kozhukharova, I.V., Borodkina, A.V., Pugovkina, N.A., Smirnova, I.S., Lyublinskaya, O.G., Fridlyanskaya, I.I., and Nikolsky, N.N., Three-dimensional compaction switches stress response programs and enhances therapeutic efficacy of endometrial mesenchymal stem/stromal cells, Front. Cell Dev. Biol., 2020, vol. 8, p. 473. https://doi.org/10.3389/fcell.2020.00473
Gargett, C.E. and Masuda, H., Adult stem cells in the endometrium, Mol. Hum. Reprod., 2010, vol. 16, p. 818. https://doi.org/10.1093/molehr/gaq061
Gorgieva, S. and Trček, J., Bacterial cellulose: production, modification and perspectives in biomedical applications, Nanomaterials, 2019, vol. 9, p. 1352. https://doi.org/10.3390/nano9101352
Guruswamy Damodaran, R. and Vermette, P., Tissue and organ decellularization in regenerative medicine, Biotech. Progress, 2018, vol. 34, p. 1494. https://doi.org/10.1002/btpr.2699
Haycock, J.W., 3D cell culture: a review of current approaches and techniques, Methods Mol. Biol., 2011, vol. 695, p. 1. https://doi.org/10.1007/978-1-60761-984-0_1
Husein, K.S. and Thiemermann, C., Mesenchymal stromal cells: current understanding and clinical status, Stem Cells, 2010, vol. 28, p. 585. https://doi.org/10.1002/stem.269
Jauković, A., Abadjieva, D., and Trivanović, D., Specificity of 3D MSC spheroids microenvironment: impact on MSC behavior and properties, Stem Cell Rev. Rep., 2020, vol. 16, p. 853. https://doi.org/10.1007/s12015-020-10006-9
Jensen, C. and Teng, Y., Is it time to start transitioning from 2D to 3D cell culture?, Front. Mol. Biosci., 2020, vol. 7, p. 33. https://doi.org/10.3389/fmolb.2020.00033
Kouroupis, D. and Correa, D., Increased mesenchymal stem cell functionalization in three-dimensional manufacturing settings for enhanced therapeutic applications, Front. Bioeng. Biotechnol., 2021, vol. 9, p. 621748. https://doi.org/10.3389/fbioe.2021.621748
Langhans, S.A., Three-dimensional in vitro cell culture models in drug discovery and drug repositioning, Front. Pharmacol., 2018, vol. 9, p. 6. https://doi.org/10.3389/fphar.2018.00006
Lee, J., Jung, H., and Park, N., Induced osteogenesis in plants decellularized scaffolds, Sci. Rep., 2019, vol. 9, p. 20194. https://doi.org/10.1038/s41598-019-56651-0
Lyublinskaya, O.G., Ivanova, J.S., Pugovkina, N.A., Kozhukharova, I.V., Kovaleva, Z.V., Shatrova, A.N., Aksenov, N.D., Zenin, V.V., Kaulin, Y.A., Gamaley, I.A., and Nikolsky, N.N., Redox environment in stem and differentiated cells: a quantitative approach, Redox Biol., 2017, vol. 12, p. 758. https://doi.org/10.1016/j.redox.2017.04.016
Meng, X., Ichim, T.E., Zhong, J., Rogers, A., Yin, Z., Jackson, J., Wang, H., Ge, W., Bogin, V., Chan, K.W., Thébaud, B., and Riordan, N.H., Endometrial regenerative cells: a novel stem cell population, J. Transl. Med., 2007, vol. 5, p. 57. https://doi.org/10.1186/1479-5876-5-57
Modulevsky, D.J., Lefebvre, C., Haase, K., Al-Rekabi, Z., and Pelling, A.E., Apple derived cellulose scaffolds for 3D mammalian cell culture, PLoS One, 2014, vol. 9, p. e97835. https://doi.org/10.1371/journal.pone.0097835
Modulevsky, D.J., Cuerrier, C.M., and Pelling, A.E., Biocompatibility of subcutaneously implanted plant-derived cellulose biomaterials, PLoS One, 2016, vol. 11, p. e0157894. https://doi.org/10.1371/journal.pone.0157894
Musina, R.A., Tarusova, O.V., Solovyova, E.V., Sukhikh, G.T., and Belyavski, A.V., Endometrial mesenchymal stem cells isolated from the menstrual blood, Kl. Tekhn. Biol. Med., 2008, vol. 145, p. 539.
Patel, A.N., Park, E., Kuzman, M., Benetti, F., Silva, F.J., and Allickson, J.G., Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation, Cell Transplant., 2008, vol. 17, p. 303. https://doi.org/10.3727/096368908784153922
Phan, N.V., Wright, T., Rahman, M.M., Xu, J., and Coburn, J.M., In vitro biocompatibility of decellularized cultured plant cell derived matrices, ACS Biomater. Sci. Eng., 2020, vol. 6, p. 822. https://doi.org/10.1021/acsbiomaterials.9b00870
Svensson, A., Nicklasson, E., Harrah, T., Panilaitis, B., Kaplan, D.L., Brittberg, M., and Gatenholm, P., Bacterial cellulose as a potential scaffold for tissue engineering of cartilage, Biomaterials, 2005, vol. 26, p. 419. https://doi.org/10.1016/j.biomaterials.2004.02.049
Hu Xinqiang, Xia Zengzilu, and Cai Kaiyong, Recent advances in 3D hydrogel culture systems for mesenchymal stem cell-based therapy and cell behavior regulation, J. Mater. Chem. B, 2022, vol. 10, p. 1486. https://doi.org/10.1039/D1TB02537F
Zack, G.W., Rogers, W.E., and Latt, S.A., Automatic measurement of sister chromatid exchange frequency, J. Histochem. Cytochem., 1977, vol. 25, p. 741. https://doi.org/10.1177/25.7.70454
Zemelko, V.I., Grinchuk, T.M., Domnina, A.P., Artzibasheva, I.V., Zenin, V.V., Kirsanov, A.A., Bichevaia, N.K., Korsak, V.S., and Nikolsky, N.N., Multipotent mesenchymal stem cells of desquamated endometrium: isolation, characterization and use as feeder layer for maintenance of human embryonic stem cell lines, Cell Tissue Biol., 2012, vol. 6, p. 1.
ACKNOWLEDGMENTS
Vertebrate Cell Culture Collection of Shared Research Facility of the Institute of Cytology, Russian Academy of Sciences (St. Petersburg), from which eMSCs were obtained, was supported by the Ministry of Education and Science of the Russian Federation, agreement no. 075-15-2021-683.
Funding
This work was supported financially by the Russian Science Foundation, project no. 22-74-10126.
Author information
Authors and Affiliations
Contributions
I.K. Kuneev and Yu.S. Ivanova (equal contribution): obtaining and preparing cellulose matrices, culturing cells, population of matrices with cells, studying the properties of cells in matrices, participating in writing the text of the article; Yu.A. Nashchekina: development of a method for coating cellulose matrices with collagen, E.K. Patronova: data processing; A.V. Sokolova: participation in the study of the properties of cells in matrices; A.P. Domnina: plan of experiments, processing and analysis of the results, participation in writing and editing the text of the article.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. The authors did not conduct experiments using animals or human beings.
Additional information
Abbreviations: eMSC—endometrial mesenchymal stem (stromal) cell; EdU—5-ethynyl-2'-deoxyuridine; PBS—phosphate buffered saline; PI—propidium iodide; SDS—sodium dodecyl.
Rights and permissions
About this article
Cite this article
Kuneev, I.K., Ivanova, Y.S., Nashchekina, Y.A. et al. Development of a Method for Three-Dimensional Culturing of Human Mesenchymal Stem (Stromal) Cells Using a Cellulose Matrix. Cell Tiss. Biol. 17, 388–397 (2023). https://doi.org/10.1134/S1990519X2304003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X2304003X