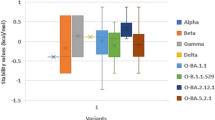
Abstract
Omicron derived lineages viz. BA.2, BA.3, BA.4 BA.5, BF.7 and XBBs show prominence with improved immune escape, transmissibility, infectivity, and pathogenicity in general. Sub-variants, XBB.1.5 and XBB.1.16 have shown rapid spread, with mutations embedded throughout the viral genome, including the spike protein. Changing atomic landscapes in spike contributes significantly to modulate host pathogen interactions and infections thereof. In the present work, we computationally analyzed the binding affinities of spike receptor binding domains (RBDs) of XBB.1.5 and XBB.1.16 towards human angiotensin-converting enzyme 2 (hACE2) compared to Omicron. We have employed simulations and binding energy estimation of molecular complexes of spike-hACE2 to assess the interplay of interaction pattern and effect of mutations if any in the binding mode of the RBDs of these novel mutants. We calculated the binding free energy (BFE) of the RBD of the Omicron, XBB.1.5 and XBB.1.16 spike protein to hACE2. We showed that XBB.1.5 and XBB.1.16 can bind to human cells more strongly than Omicron due to the increased charge of the RBD, which enhances the electrostatic interactions with negatively charged hACE2. The per-residue decompositions further show that the Asp339His, Asp405Asn and Asn460Lys mutations in the XBBs RBD play a crucial role in enhancing the electrostatic interactions, by acquiring positively charged residues, thereby influencing the formation/loss of interfacial bonds and thus strongly affecting the spike RBD-hACE2 binding affinity. Simulation results also indicate less interference of heterogeneous glycans of XBB.1.5 spike RBD towards binding to hACE2. Moreover, despite having less interaction at the three interfacial contacts between XBB S RBD and hACE2 compared to Omicron, variants XBB.1.5 and XBB.1.16 had higher total binding free energies (ΔGbind) than Omicron due to the contribution of non-interfacial residues to the free energy, providing insight into the increased binding affinity of XBB1.5 and XBB.1.16. Furthermore, the presence of large positively charged surface patches in the XBBs act as drivers of electrostatic interactions, thus support the possibility of a higher binding affinity to hACE2.







Similar content being viewed by others
References
Classification of Omicron (B.1.1.529) (2022) : SARS-CoV-2 Variant of Concern, (n.d.). https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
Kumar S, Karuppanan K, Subramaniam G (2022) Omicron (BA.1) and sub-variants (BA.1.1, BA.2, and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: a comparative sequence and structural-based computational assessment. J Med Virol 94(10):4780–4791. https://doi.org/10.1002/jmv.27927
Singh, Jaikee et al (2023) Neohesperidin and spike RBD interaction in omicron and its sub-variants: in silico, structural and simulation studies. Comput Biol Medicine 152: 106392. https://doi.org/10.1016/j.compbiomed.2022.106392
Hachmann NP, Miller J, Collier AY et al Neutralization escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med, 387(1), 86–88. https://doi.org/10.1056/NEJMc2206576
Wang Q, Guo Y, Iketani S et al Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature, 608(7923), 603–608. https://doi.org/10.1038/s41586-022-05053-w
Callaway E (2023) Coronavirus variant XBB.1.5 rises in the United States - is it a global threat? Nature 613(7943):222–223. https://doi.org/10.1038/d41586-023-00014-3
World Health Organization (WHO). Technical Advisory Group on Virus Evolution (TAG-VE).25January2023. https://www.who.int/docs/defaultsource/coronaviruse/24feb2023_xbb15_rapid_risk_assessment.pdf
Centers for Disease Control and Prevention (CDC). COVID data tracker. March 19, 2023. Available at: https://covid.cdc.gov/covid-data-tracker/#variant-proportions
Wang Q, Iketani S, Li Z et al (2023) Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186(2):279–286e8. https://doi.org/10.1016/j.cell.2022.12.018
Ao D, He X, Hong W, Wei X (2023) The rapid rise of SARS-CoV-2 Omicron subvariants with immune evasion properties: XBB.1.5 and BQ.1.1 subvariants. MedComm (2020). ;4(2):e239. Published 2023 Mar 15. https://doi.org/10.1002/mco2.239
Sugano A, Kataguchi H, Ohta M et al (2023) SARS-CoV‐2 Omicron XBB.1.5 may be a cautionary variant by in silico study. bioRxiv Posted January 25. https://doi.org/10.1101/2023.01.18.524660bioRxiv
Yue C, Song W, Wang L et al (2023) ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect Dis 23(3):278–280. https://doi.org/10.1016/S1473-3099(23)00010-5
Uriu K, Ito J, Zahradnik J et al (2023) Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect Dis 23(3):280–281. https://doi.org/10.1016/S1473-3099(23)00051-8
Liang B et al (2023) SARS-CoV-2 spike protein Post-Translational Modification Landscape and its impact on protein structure and function via computational prediction. Res (Washington D C) 6:0078. https://doi.org/10.34133/research.0078
Harbison AM et al (2021) Fine-tuning the spike: role of the nature and topology of the glycan shield in the structure and dynamics of the SARS-CoV-2 S. Chem Sci vol 13:2 386–395 25 Nov. https://doi.org/10.1039/d1sc04832e
Fazekas Z et al (2022) Omicron binding Mode: Contact Analysis and Dynamics of the Omicron receptor-binding domain in Complex with ACE2. J Chem Inform Model vol 62:3844–3853. https://doi.org/10.1021/acs.jcim.2c00397
Singh JK et al (2023) Is BF.7 more infectious than other Omicron subtypes: insights from structural and simulation studies of BF.7 spike RBD variant. Int J Biol Macromol 238:124154. https://doi.org/10.1016/j.ijbiomac.2023.124154
Han P, Li L, Liu S et al (2022) Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell 185(4):630–640e10. https://doi.org/10.1016/j.cell.2022.01.001
Jo S, Kim T, Iyer VG, Im W (2008) CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem 29(11):1859–1865. https://doi.org/10.1002/jcc.20945
Jo S et al (2011) Glycan reader: automated sugar identification and simulation preparation for carbohydrates and glycoproteins. J Comput Chem vol 32:3135–3141. https://doi.org/10.1002/jcc.21886
Park SJ, Lee J, Patel DS et al (2017) Glycan reader is improved to recognize most sugar types and chemical modifications in the Protein Data Bank. Bioinformatics 33(19):3051–3057. https://doi.org/10.1093/bioinformatics/btx358
Park SJ, Lee J, Qi Y et al (2019) CHARMM-GUI glycan modeler for modeling and simulation of carbohydrates and glycoconjugates. Glycobiology 29(4):320–331. https://doi.org/10.1093/glycob/cwz003
Shajahan A et al (2021) “Comprehensive characterization of N- and O- glycosylation of SARS-CoV-2 human receptor angiotensin converting enzyme 2.” Glycobiology vol. 31,4 : 410–424. https://doi.org/10.1093/glycob/cwaa101
Watanabe Y et al (2020) Site-specific glycan analysis of the SARS-CoV-2 spike. Sci (New York N Y) vol 369(6501):330–333. https://doi.org/10.1126/science.abb9983
Abraham MJ et al (2015) High performance molecular simulations through multi-level parallelism from laptops to supercomputer. SoftwareX 1–2:19–25
Huang J et al (2017) CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat methods vol 14(1):71–73. https://doi.org/10.1038/nmeth.4067
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Hess B, Bekker H, Berendsen HJC, Fraaije JG (1997) E. M. LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Turner PJ Center for Coastal and Land-Margin Research, Oregon Graduate Institute of Science and Technology; Beaverton, OR: 2005. XMGRACE, Version 5.1.19.
Pettersen EF et al (2004) UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Laskowski RA, Swindells MB, LigPlot+ (2011) Multiple ligand-protein Interaction Diagrams for Drug Discovery. J Chem Inf Model 51:2778–2786
Kumari R, Kumar R, Open-Source DD, Consortium, Lynn A (2014) g_mmpbsa–a GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 54(7):1951–1962. https://doi.org/10.1021/ci500020m
Du X, Li Y, Xia YL et al (2016) Insights into protein-ligand interactions: mechanisms, models, and methods. Int J Mol Sci 17(2):144. https://doi.org/10.3390/ijms17020144. Published 2016 Jan 26
Foloppe N, Hubbard R (2006) Towards predictive ligand design with free-energy based computational methods? Curr Med Chem 13(29):3583–3608. https://doi.org/10.2174/092986706779026165
Levy RM et al “On the nonpolar hydration free energy of proteins: surface area and continuum solvent models for the solute-solvent interaction energy.” J Am Chem Soc vol. 125,31 (2003): 9523–9530. https://doi.org/10.1021/ja029833a
Levitt M et al (1985) Protein normal-mode dynamics: trypsin inhibitor, crambin, ribonuclease and lysozyme. J Mol biology vol 181(3):423–447. https://doi.org/10.1016/0022-2836(85)90230-x
Laskowski RA, Jabłońska J, Pravda L, Vařeková RS, Thornton JM, PDBsum (2018) Structural summaries of PDB entries. Protein Sci 27:129–134. https://doi.org/10.1002/pro.3289
Nguyen HL et al (2022) SARS-CoV-2 Omicron variant binds to human cells more strongly than the wild type: evidence from Molecular Dynamics Simulation. J Phys Chem B vol 126:4669–4678. https://doi.org/10.1021/acs.jpcb.2c01048
Mehdipour AR, Hummer G (2021) Dual nature of human ACE2 glycosylation in binding to SARS-CoV-2 spike. Proc Natl Acad Sci U S A 118(19):e2100425118. https://doi.org/10.1073/pnas.2100425118
Acknowledgements
The authors acknowledge the support of the Internet of Things (IoT) and Data Communication Lab; Multiscale Simulation and Research Center (MSRC) Manipal University Jaipur for computational calculations. JKS acknowledges the support of senior research fellowship from ICMR, India.
Funding
The work was financially supported by Science and Engineering Research Board (SERB), India Grant CRG/2020/002008 awarded to S.K.S.
Author information
Authors and Affiliations
Contributions
formal analysis, investigation- JKS, JS, SKS; writing-review and editing- JKS, SKS; conceptualization, supervision, funding acquisition- SKS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, J.K., Singh, J. & Srivastava, S.K. Investigating the role of glycans in Omicron sub-lineages XBB.1.5 and XBB.1.16 binding to host receptor using molecular dynamics and binding free energy calculations. J Comput Aided Mol Des 37, 551–563 (2023). https://doi.org/10.1007/s10822-023-00526-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-023-00526-0