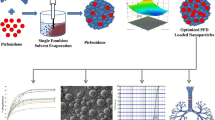
Abstract
Purpose
The purpose of this study was to develop inhalable pirfenidone (PFD) nanoparticles for the delivery of the medicine to specific alveolar epithelial cells (AECs) in the idiopathic pulmonary fibrosis (IPF) treatment. PFD has major problems like low oral route absorption due to the presence of food, faster elimination half-life, and potent phototoxicity. PFD-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles provide enhanced release of drug and reduce its toxicity, dose, and dosing frequency by targeting the AECs.
Methods
The PFD-loaded PLGA NPs were prepared by a single emulsion solvent evaporation method. The preliminary studies were carried out with process parameters like the selection of organic phase, effect of organic phase volume, effect of stabilizer (PVA) concentration, drug:polymer ratio, and sonication time. The effects of two formulation variables including the drug:polymer ratio and surfactant concentration were examined by using 32 full factorial design applied to get optimized formulation. The final product was lyophilized and made free-flowing powder formulation for pulmonary administration.
Results
Optimized PFD-loaded PLGA NPs showed initial burst release followed by sustained up to 71.19 ± 5.39% at 24 h. The particle size, polydispersity index, zeta potential, and % entrapment efficiency were found to be 523 ± 6.42 nm, 0.183 ± 0.011, 1.62 ± 0.22 mV, and 44.14 ± 3.74% respectively.
Conclusions
There was no significant aggregation of PFD-loaded PLGA NPs during 6 months of stability study and spherical shape nanoparticle with smooth surface as per SEM study. Hence, PFD-loaded PLGA NPs can be a suitable alternative to the currently available therapy.
Graphical Abstract











Similar content being viewed by others
Data Availability
All data and material are available upon request. Data attached as supplimentary material too.
Abbreviations
- AECs:
-
Alveolar epithelial cells
- ANOVA:
-
Analysis of variance
- DCM:
-
Dichloromethane
- DoE:
-
Design of the experiment
- DSC:
-
Differential scanning calorimetry
- EE:
-
Entrapment efficiency
- FDA:
-
Food and Drug Administration
- FTIR:
-
Fourier transform infrared spectroscopy
- GSD:
-
Geometric standard deviation
- IPF:
-
Idiopathic pulmonary fibrosis
- MMAD:
-
Mass median aerodynamic diameter
- NPs:
-
Nanoparticles
- PFD:
-
Pirfenidone
- PLGA:
-
Poly(D,L-lactic-co-glycolic acid)
- PVA:
-
Polyvinyl alcohol
- PI:
-
Polydispersity index
- SEM:
-
Scanning electron microscopy
- TNF-α:
-
Tumor necrosis factor-α
- TGF-β:
-
Transforming growth factor-β
- ZP:
-
Zeta potential
References
Raghu G, Richeldi L. Current approaches to the management of idiopathic pulmonary fibrosis. Respir Med. 2017;129:24–30.
Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–23.
Zhang B, Ding Z, Dong J, Lin F, Xue Z, Xu J. Macrophage-mediated degradable gelatin-coated mesoporous silica nanoparticles carrying pirfenidone for the treatment of rat spinal cord injury. Nanomedicine. 2021;37:102420.
Abnoos M, Mohseni M, Mousavi SAJ, Ashtari K, Ilka R, Mehravi B. Chitosan-alginate nano-carrier for transdermal delivery of pirfenidone in idiopathic pulmonary fibrosis. Int J Biol Macromol. 2018;118:1319–25.
Jose A, Mandapalli PK, Venuganti VK. Liposomal hydrogel formulation for transdermal delivery of pirfenidone. J Liposome Res. 2016;26(2):139–47.
Gaul R, Ramsey JM, Heise A, Cryan S-A, Greene CM. Nanotechnology approaches to pulmonary drug delivery: targeted delivery of small molecule and gene-based therapeutics to the lung. Design of nanostructures for versatile therapeutic applications: Elsevier; 2018. p. 221–53.
Pardeshi S, Patil P, Rajput R, Mujumdar A, Naik J. Preparation and characterization of sustained release pirfenidone loaded microparticles for pulmonary drug delivery: Spray drying approach. Drying Technol. 2020;39(3):337–47.
Choi J-S, Seo K, Yoo J-W. Recent advances in PLGA particulate systems for drug delivery. J Pharm Investig. 2012;42(3):155–63.
Saha D, Kumar S, Ray D, Kohlbrecher J, Aswal VK. Role of physicochemical parameters associated with the hydrophobic vs. amphiphilic biodegradable polymer nanoparticles formation. J Mol Liq. 2020;318:113977.
Meng H, Xu Y. Pirfenidone-loaded liposomes for lung targeting: preparation and in vitro/in vivo evaluation. Drug Des Dev Ther. 2015;9:3369.
Wu C, Or PW, Chong JIT, K. Pathirage Don IK, Lee CHC, Wu K, et al. Controllable release of pirfenidone by polyvinyl alcohol film embedded soft contact lenses in vitro and in vivo. Drug Delivery. 2021;28(1):634–41.
Chaudhary SA, Patel DM, Patel JK, Patel DH. Solvent emulsification evaporation and solvent emulsification diffusion techniques for nanoparticles. Emerging Technologies for Nanoparticle Manufacturing: Springer; 2021. p. 287–300.
Kızılbey K. Optimization of rutin-loaded PLGA nanoparticles synthesized by single-emulsion solvent evaporation method. ACS Omega. 2019;4(1):555–62.
Sadozai SK, Khan SA, Karim N, Becker D, Steinbrück N, Gier S, et al. Ketoconazole-loaded PLGA nanoparticles and their synergism against Candida albicans when combined with silver nanoparticles. J Drug Deliv Sci Technol. 2020;56:101574.
Elsewedy HS, Dhubiab BEA, Mahdy MA, Elnahas HM. Development, optimization, and evaluation of PEGylated brucine-loaded PLGA nanoparticles. Drug Delivery. 2020;27(1):1134–46.
Jahromi LP, Ghazali M, Ashrafi H, Azadi A. A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles. Heliyon. 2020;6(2):e03451.
Debnath SK, Saisivam S, Omri A. PLGA ethionamide nanoparticles for pulmonary delivery: Development and in vivo evaluation of dry powder inhaler. J Pharm Biomed Anal. 2017;145:854–9.
Shah P, Sarolia J, Vyas B, Wagh P, Ankur K, Kumar MA. PLGA nanoparticles for nose to brain delivery of clonazepam: formulation, optimization by 32 Factorial design, in vitro and in vivo evaluation. Current Drug Delivery. 2021.
Kumari N, Bhattacharya B, Roy P, Michalchuk AA, Emmerling F, Ghosh A. Enhancing the pharmaceutical properties of pirfenidone by mechanochemical cocrystallization. Cryst Growth Des. 2019;19(11):6482–92.
Vineeth P, Vadaparthi P, Kumar K, Babu BDJ, Rao AV, Babu KS. Influence of organic solvents on nanoparticle formation and surfactants on release behaviour in-vitro using costunolide as model anticancer agent. Int J Pharm Pharm Sci. 2014;6(4):638–45.
Beck-Broichsitter M. Solvent impact on polymer nanoparticles prepared nanoprecipitation. Colloids Surf A Physicochem Eng Asp. 2021:126928.
Madani F, Esnaashari SS, Mujokoro B, Dorkoosh F, Khosravani M, Adabi M. Investigation of effective parameters on size of paclitaxel loaded PLGA nanoparticles. Adv Pharm Bull. 2018;8(1):77.
Najlah M, Ahmed Z, Iqbal M, Wang Z, Tawari P, Wang W, et al. Development and characterisation of disulfiram-loaded PLGA nanoparticles for the treatment of non-small cell lung cancer. Eur J Pharm Biopharm. 2017;112:224–33.
Huang W, Zhang C. Tuning the size of poly (lactic-co-glycolic acid)(PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol J. 2018;13(1):1700203.
Esnaashari SS, Amani A. Optimization of noscapine-loaded mPEG-PLGA nanoparticles and release study: a response surface methodology approach. J Pharm Innov. 2018;13(3):237–46.
Patel J, Amrutiya J, Bhatt P, Javia A, Jain M, Misra A. Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J Microencapsul. 2018;35(2):204–17.
Otroj M, Taymouri S, Varshosaz J, Mirian M. Preparation and characterization of dry powder containing sunitinib loaded PHBV nanoparticles for enhanced pulmonary delivery. J Drug Delivery Sci Technol. 2020;56:101570.
Baghaei B, Saeb MR, Jafari SH, Khonakdar HA, Rezaee B, Goodarzi V, et al. Modeling and closed-loop control of particle size and initial burst of PLGA biodegradable nanoparticles for targeted drug delivery. J Appl Polym Sci. 2017;134(33):45145.
Nair A, Khunt D, Misra M. Application of quality by design for optimization of spray drying process used in drying of risperidone nanosuspension. Powder Technol. 2019;342:156–65.
Khuroo T, Verma D, Khuroo A, Ali A, Iqbal Z. Simultaneous delivery of paclitaxel and erlotinib from dual drug loaded PLGA nanoparticles: formulation development, thorough optimization and in vitro release. J Mol Liq. 2018;257:52–68.
Shkodra-Pula B, Grune C, Traeger A, Vollrath A, Schubert S, Fischer D, et al. Effect of surfactant on the size and stability of PLGA nanoparticles encapsulating a protein kinase C inhibitor. Int J Pharm. 2019;566:756–64.
Acknowledgements
The authors would like to thank Dr. Praful Bharadiya and Dr. Mihir Raval for their helpful comments. The authors are also thankful to Meck Pharmaceuticals & Chemicals Private Limited and ZCL Chemicals Ltd., Gujarat, India, for providing respective gift samples.
Funding
The Department of Technical Education, Government of Gujarat, India, provided the financial assistance (minor research grant) provided under the SSIP (Student Startup & Innovation Policy) scheme and KS Patel Centre for Entrepreneurship, RK University, for this project.
Author information
Authors and Affiliations
Contributions
The authors Ms. Kiran Dudhat and Dr. Harsha Patel are considerably subsidized to the writing of this research article. Ms. Kiran Dudhat, who is the first author, has played a critical part in this article. Development, design, and evaluation of the PFD-loaded PLGA nanoparticles were by her. Dr. Harsha Patel and Dr. Dhaval Mori provided guidance and reviewed the article judgmentally for its knowledgeable content and assisted in the concluding endorsement of the version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dudhat, K.R., Patel, H.V. & Mori, D.D. Design, Development, and In Vitro Characterization of Pirfenidone-Loaded Biodegradable Nanoparticles for Idiopathic Pulmonary Fibrosis. J Pharm Innov 18, 1908–1925 (2023). https://doi.org/10.1007/s12247-023-09763-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09763-0